
Baebies Inc. Raises $10 Million, Sells 2 Millionth Test
Durham-based Baebies Inc., a developer of products and services for newborn screening and pediatric testing, has closed on $10 million in Series B financing.
The funding round was led by BOE Technology Group of Beijing, along with funding from family offices and continued support from current investors and the North Carolina Biotechnology Center, the company announced in a news release.
“Baebies’ digital microfluidic technology is the future of newborn screening methods, and we’re proud to collaborate in technology development and play a role in bringing this technology to the global market,” said Lijun Mao, general manager of BOE Sensor Technology Co.
Wei Wu, Ph.D., healthcare investment director of BOE Ventures, said, “As new technologies arise in healthcare, we want to back the most innovative and promising companies. Baebies has demonstrated rapid growth in the company’s product pipeline and team. The company is a tremendous fit with our Healthcare business, which is committed to offering people-oriented healthcare services by developing mobile healthcare, digital hospitals, regenerative medicine and healthcare park solutions.”
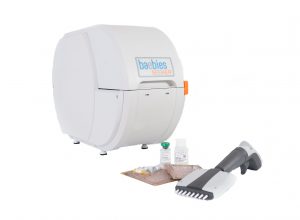
Baebies also announced the shipment of its 2 millionth test for its SEEKER system, a benchtop workstation that tests dried bloodspot specimens for enzyme levels associated with Gaucher, Fabry, Pompe and mucopolysaccharidosis type 1 diseases. If not detected and treated, these rare genetic metabolic disorders may cause organ damage, neurological disability or death.
SEEKER was cleared by the U.S. Food and Drug Administration earlier this year and remains the only newborn screening platform authorized for testing for lysosomal storage disorders. The system also received CE Mark approval in June allowing the sale of SEEKER in Europe and other countries.
 “This has been an exciting year for Baebies,” said Richard West, the company’s co-founder and chief executive officer. In addition to gaining regulatory clearance for SEEKER, he said the company has grown to 60 employees and gained a global distribution partnership with Labsystems Diagnostics to expand the product offerings in the newborn screening market.
“This has been an exciting year for Baebies,” said Richard West, the company’s co-founder and chief executive officer. In addition to gaining regulatory clearance for SEEKER, he said the company has grown to 60 employees and gained a global distribution partnership with Labsystems Diagnostics to expand the product offerings in the newborn screening market.
“We are getting closer to our goal of expanding newborn screening and pediatric testing worldwide to the millions of babies that don’t have access to it,” he said.
Co-founder and President Vamsee Pamula, Ph.D., said, “When Richard West and I founded this company in 2014, we set a goal that by 2018 we would provide 2 million chances for a healthy start. Early intervention is critical, and we are thrilled to have reached this milestone early. We never lose sight of the fact that every test shipped holds the potential to save a life.”
Baebies raised $13 million in equity financing in 2015 to commercialize its newborn-screening technology. Investors include Rex Health Ventures, DUMAC, Cunning Capital, Triad LLC, the Duke Angel Network, Charleston Angel Partners and members of Baebies’ executive team.
The financing followed a $500,000 Strategic Growth Loan from the North Carolina Biotechnology Center in 2014.
Baebies licensed its microfluidics technology from Illumina, a global genetic sequencing and genotyping company based in San Diego. Illumina, which has an equity stake in Baebies, acquired the technology from Morrisville-based Advanced Liquid Logic in 2013 for $96 million.
Advanced Liquid Logic was a Duke University spinout company co-founded by Pamula and led by West, so the technology has come full circle with Baebies.
