
UNC Spinout Developing Device for Better Chemo Delivery
Pancreatic cancer is an elusive and deadly disease.
It often has no initial symptoms, and there are no reliable tests for early detection. The mortality rate is 75 percent within a year of diagnosis, and the five-year survival rate is only 8.5 percent. And for those whose tumors have spread too far to be surgically removed, life expectancy is only six months to a year.
 “It’s just devastating,” says entrepreneur Tony Voiers. “These patients really have so few options.”
“It’s just devastating,” says entrepreneur Tony Voiers. “These patients really have so few options.”
Voiers’ company, Advanced Chemotherapy Technologies (ACT) of Raleigh, hopes to change that dim calculus for cancer patients. It is developing an implantable device to infuse chemotherapy drugs directly into the pancreas, targeting difficult-to-reach tumors while largely sparing surrounding tissues, organs and blood vessels.
More-precise drug delivery could shrink tumors enough for surgeons to remove them, the best treatment option, says Voiers, the company’s chief executive officer. While not always curative, that improvement could extend those patients’ life expectancy.
“To give people more time and a better quality of life, that’s what we’re aiming for,” Voiers says.

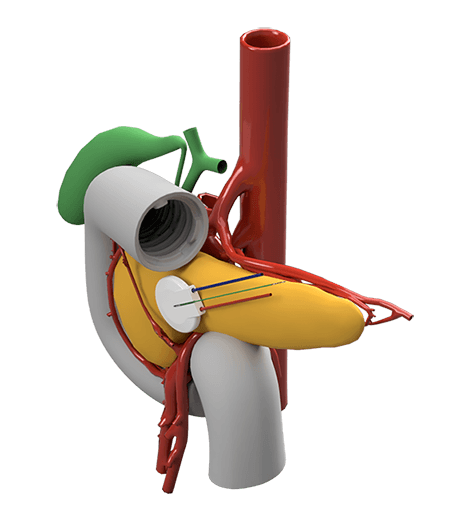
showing its position on the pancreas (below) -- ACT photos
The device, about the size of a quarter, would be implanted in the pancreas with electrical leads running to the abdomen. The device would drive chemotherapy into the tumor using electrical currents that pass through the drug solution, a process called iontophoresis.
In a pre-clinical study at the University of North Carolina at Chapel Hill, a smaller version of the device was tested successfully on human pancreatic cancers implanted in special mice. The mice had been engineered so their immune systems would not reject the patient-derived tumors.
In the mice treated systemically with gemcitabine, an approved chemotherapy drug for pancreatic cancer, the drug slowed the rate of tumor growth. However, tumors still grew about 2.5 times their original size.
In the placebo mice receiving no drug, tumors increased to several times their original size.
But in the mice receiving the drug directly into the pancreas through ACT’s device, tumors shrank by about 40 percent.
“That’s what got me into the company,” Voiers says. “That was a very impressive reduction from my standpoint. That’s what we’re working to replicate in humans.”
ACT plans to begin first-in-human testing of the device next year at UNC. Up to 12 pancreatic cancer patients will receive gemcitabine through the device in a Phase 1 clinical trial to establish safety.
If that trial and subsequent Phase 2 and 3 trials go well, it should take five to seven years to bring the device to market, Voiers says.
"A drug development type of timeline"
“There’s a long way to go before testing in humans would lead to an approval,” he says. “It’s a drug development type of timeline.”
In addition to pancreatic cancer, the device could be used to treat other solid-tumors such as sarcomas, head and neck, and breast cancer, Voiers says, because it is compatible with most chemotherapy drugs.
“Anything that’s a polar molecule in solution can be carried by electrical current,” he says. “We have a long list of chemotherapies, and indications for those, that we have looked at.”
The device was developed at UNC’s School of Medicine by collaborating scientists Jen Jen Yeh, M.D., and Joseph DeSimone, Ph.D. Yeh is associate professor and vice chair of research in the Department of Surgery, and DeSimone is a chancellor's eminent professor of chemistry at UNC and the William R. Kenan Jr. Distinguished Professor of Chemical Engineering at N.C. State University and of Chemistry at UNC. DeSimone is also co-founder and CEO of Carbon3D, a three-dimensional printing company, in California’s Silicon Valley.
James Byrne, an M.D./Ph.D. graduate student in DeSimone’s lab, did primary work on the device for his Ph.D. thesis. He constructed the device and examined its ability to deliver chemo drugs effectively to pancreatic cancer tumors, as well as two types of breast cancer tumors.
ACT holds exclusive rights to the patented technology.
NCBiotech funding support called "a godsend"
The company is financed by angel investments and federal grant funding from the Small Business Innovation and Research (SBIR) Program. It also received a $250,000 Small Business Research Loan from the North Carolina Biotechnology Center in 2017 to scale the device from mice to humans.

The Biotech Center funding “is just a godsend for a small startup company like this,” Voiers says.
The company is a finalist in an annual company-pitch competition sponsored by MedTech Innovator, a global, nonprofit accelerator for medical device, digital health and diagnostic companies. ACT won the People’s Choice Award at a regional competition in April at the Biotech Center and will advance in the national competition.
The winner will be announced in September at the MedTech Conference, sponsored by the Advanced Medical Technology Association (AdvaMed), in Philadelphia. The winning company will receive a $500,000 award.
ACT was formed in 2014, and Voiers joined the company in 2016. He and chief technology officer William Daunch are the company’s only two employees thus far.
Voiers, a graduate of North Carolina State University, is a medical device entrepreneur who was CEO and co-founder of Novocor Medical Systems in Durham before joining ACT. Previously he was an executive at Ethicon, Closure Medical and Abbott.
