
AskBio’s Co-Founder on Hitting $4B Bayer Deal ‘Milestones,’ Her Side Hustle Foundation, and Living with Lupus
(Editor’s note: This is the second in a series of articles to be published from the forum. The first one is here and the third one here.)
Seventeen months after negotiating a whopping $4 billion buyout deal with German pharma giant Bayer, Asklepios BioPharmaceutical (AskBio) CEO and co-founder Sheila Mikhail says the pioneering gene therapy company is hitting all its targets.
“COVID has not set us back,” said Mikhail, shortly after stepping off stage as part of an all-star speaker lineup at LaunchBio’s Invest in Cures forum held at the North Carolina Biotechnology Center last week.
“We're doing what we promised.”

Headquartered in the heart of the Research Triangle, AskBio uses an adeno-associated virus-based (AAV) gene therapy platform to develop potential therapies for inherited disorders.
Since its sale in late 2020 – involving $2 billion upfront and another $2 billion in “milestone payments” -- Mikhail said it’s acquired a 308,000-square-foot manufacturing site in San Sebastian, Spain to produce and sell doggybone DNA (dbDNA) technology into the AAV market.
It’s also added clinical programs in Parkinson’s disease and multiple system atrophy, and doubled its workforce to 600 employees, “probably [reaching] close to 1000 by the end of the year.”
“Bottom line, we’re hitting all the different milestones,” she said. “We keep acquiring companies and programs.”
It’s a long way from where Mikhail first started, launching AskBio with gene therapy pioneer Jude Samulski and UNC Professor Xiao Xiao more than 21 years ago in Chapel Hill, with little more than a handful of grants and a vision to erase genetic disease.

In that time, Mikhail, a lawyer by trade, orchestrated two sales of AskBio spinoffs to Pfizer for its Duchenne Muscular Dystrophy therapeutic currently in Phase 3 and to Takeda (Baxter) for its Hemophilia B therapeutic. She also guided the licensing agreement for self-complementary vector technology to Novartis that proved essential for the FDA approval of the gene therapy Zolgensma, to treat Spinal Muscular Atrophy (SMA); as well as negotiated key acquisitions, including Synpromics, Brain Neuropathy Bio, BrainVectis, Viralgen (CDMO) and TAAV (CDMO).
Early work by Samulski at AskBio and related entities was supported by grants and loans from the Biotechnology Center totaling about $1 million. The support included a $250,000 grant that helped recruit Samulski, the first scientist to clone AAV, to the University of North Carolina at Chapel Hill School of Medicine from the University of Pittsburgh in 1993. He directed UNC’s Gene Therapy Center for 25 years.
Today, AskBio’s portfolio includes pre-clinical and clinical-stage development candidates to treat neuromuscular, central nervous system, cardiovascular and metabolic diseases such as Pompe disease, Parkinson’s and congestive heart failure, as well as out-licensed clinical candidates for hemophilia and Duchenne muscular dystrophy.
Her side hustle
But as Mikhail is quick to point, it’s never been about profits.
“I'm not driven by money. I never have been,” she said. Instead, it’s always been about saving lives and changing the world.
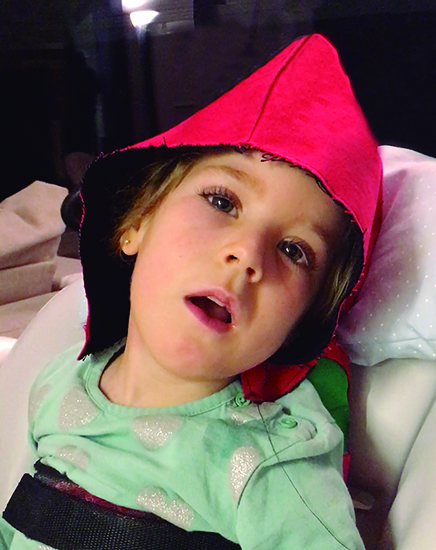
received gene therapy surgery in Varsovia’s Brodno Hospital
courtesy of the Columbus Children’s Foundation.
In 2017, she and Samulski partnered with longtime collaborators, Columbus Venture Partners, to launch the Columbus Children’s Foundation in Valencia, Spain, with U.S. headquarters in Chapel Hill. Funded largely by AskBio’s sale of Bamboo Therapeutics to Pfizer in 2016 and the most recent Bayer deal, its mission is to fund treatments for children with ultra-rare diseases.
“We don’t want our technology to only be focused on disease states where there's a large enough patient population to be profitable,” she told the 100-strong crowd gathered at the forum. “We thought that was morally wrong, to be quite blunt.”
Among its projects, the group is working to promote access to a gene therapy for an extremely rare disorder called aromatic amino acid decarboxylase (AADC) deficiency, known more commonly as “pediatric Parkinson’s.”
Affecting roughly 130 children worldwide, it’s a debilitating genetic disorder that affects the brain’s ability to produce neurotransmitters, specifically dopamine and serotonin. The disease typically presents during the first year of life. Sufferers struggle to reach milestones, such as walking and talking, and many require feeding tubes and breathing assistance.

Clinical studies have shown the therapeutic benefit in applying AAV-mediated gene therapy in such cases. But it doesn’t come cheap. Each treatment comes with a $70,000 price tag.
To date, the foundation has treated close to 30 patients, free of charge at various locations, including the University of California in San Francisco and the Interventional Neurotherapy Centre in Brodno, Poland.
Among them is Irai, from Barcelona, Spain, profiled on the foundation’s website, who presented with AADC a year her birth. She could barely sleep even three hours at a time and lived in constant pain, unable to move or speak. She underwent the complex gene therapy in Poland in May 2019.
Fast-forward to today: The 7-year-old is “walking, talking and laughing as a thriving pre-school student.”
“The treatment has incredible transformative impact,” Mikhail said.
Living with Lupus
Even as Mikhail tirelessly pushes to find cures for others, she’s been living with her own rare genetic disease.
At age 40, Mikhail was diagnosed with systemic lupus erythematosus (SLE), an autoimmune disease in which the body's immune system mistakenly attacks healthy tissue in many parts of the body. It’s a complex disease, predominantly affecting Latina and Black women, and is likely triggered by several interacting factors including inherited genes, experts say. There is no cure.
Mikhail said she suffered symptoms – including hair loss, fatigue, joint pain -- for at least a decade prior to her diagnosis. One doctor told her that “it was a figment of my imagination,” she said.
“When they finally diagnosed me, I was pretty sick. My kidneys were in jeopardy, and I had inflammation around my heart.”
In the early days, she explored gene therapy as a possible solution, but it wasn’t an option. Instead, she relies on the standard of care, hydrochloroquine. It’s an antimalarial drug that has proven to be effective for lupus management and, most recently, preventing COVID-19 mortality.
To date, Mikhail said she’s responded positively to treatment with only a few “serious flareups.” “I still must do certain things, such as walk and stretch every day, to manage the joint stiffness. But I’ve managed pretty well.”
She’s also committed to raising awareness about the disorder. That includes pushing for “more equitable distribution of capital” to fight “pockets of neglect” among rare diseases that primarily affect marginalized communities, which lupus comes under, she said.
“I want to give an example to other lupus patients that it is possible to live with the disease and have a full life,” she said. “When I was diagnosed, I was told that I would have to cut back on my work and then I could not do as many things. But that just wasn’t true.”
Meanwhile, she remains focused on AskBio’s work and the other areas where she can make a difference.
“I can have impact on a wide variety of different diseases, just not my own.”
