
Lucerno Dynamics Delivers Quality Control Solution for Nuclear Medicine Injections

Ron Lattanze, CEO of Lucerno Dynamics in Cary, believes there should be better quality control with nuclear medicine injections, such as those administered for PET/CT scans.
The Lara System, Lucerno’s noninvasive medical device, helps clinicians detect “infiltrations,” which are incidents that occur when radioactive material is mistakenly injected into the soft tissue of a patient’s arm instead of the intended vein.
The injection’s nuclear medicine is designed to accumulate at possible problem areas in a patient’s body. After the injection and a waiting period, scans are taken and turned into images for doctors to reference as they make diagnoses, determine treatment plans and monitor responses to therapy.
“Infiltrations are bad in many ways,” said Lattanze. “Depending on the severity of the infiltration, a patient’s soft tissue can be exposed to a high dose of unnecessary radiation. Also, large infiltrations can compromise the quality and quantification of the images that physicians use to guide patient care.”
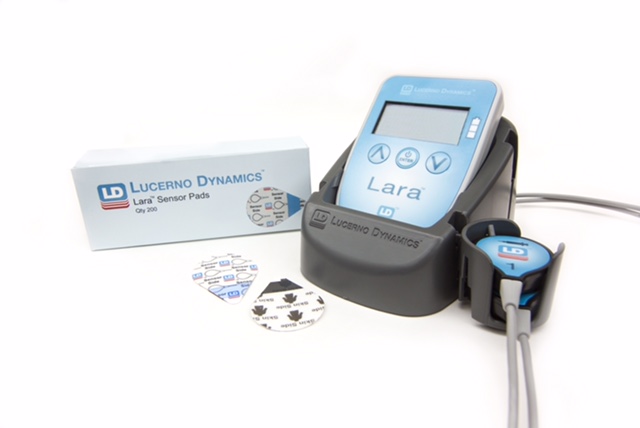
Lucerno’s Lara System monitors nuclear medicine injections quickly, safely and inexpensively. In addition, the device identifies possible contributing factors for the infiltrations such as techniques, tools, equipment positioning and patient characteristics. Hospitals and clinics that use the Lara System can review the possible contributing factors, make necessary and often simple changes, and reduce their infiltrations.
Lucerno concluded the largest nuclear medicine injection quality improvement project with the Lara System with over 5,000 patients at seven hospitals and centers in 2018 and found an aggregated infiltration rate of 6.2% for PET/CT scans. Lattanze believes hospitals and centers should have an infiltration rate of less than 1% and that this is a realistic goal.
Jackson W. Kiser, M.D., medical director of molecular imaging at Carilion Clinic in Roanoke, Va., participated in the quality improvement project. “The Lara System has been instrumental in helping us understand when infiltrations occur and why they are occurring,” said Kiser. “We have seen a striking change in our infiltration rates and they continue to stay as low as possible.”
Moving forward with commercialization and funding
Lattanze said Lucerno looks forward to partnering with nuclear medicine companies as it ramps up commercialization of the Lara System. A few hospitals in the United States and Australia are using the device. The company has plans to make it commercially available in 12 European countries in the near future.

The company, founded in 2011, was awarded a $47,000 NC IDEA grant in 2012. In early 2014 the North Carolina Biotechnology Center awarded Lucerno a $150,000 loan to help it develop and test its technology, though the company only used half of it. In 2016 the company raised $6.34 million in Series A funding.
Now Lucerno is in the midst of raising Series B funding, which Lattanze said will carry the company through becoming profitable on its own. New funding will help Lucerno build partnerships with companies to further develop its technology for more applications.
Advocating for change to nuclear medicine public policy
Lucerno plays an active role in driving public policy change regarding nuclear medicine procedures and patient safety.
The U.S. Nuclear Regulatory Commission (NRC) requires nuclear medicine providers report medical events that result in unintended radiation exposure to the patient’s tissue over a certain limit. But due to a loophole, infiltrations have been exempt from this reporting requirement since 1980. As a result, neither patients nor physicians are notified when a patient is exposed to radiation that far exceeds NRC limits.
“Nuclear medicine has never been required to report infiltrations,” said Lattanze. “Thus, injection quality improvement is not a priority and there’s no transparency.”
Lattanze said that if hospitals and clinics were required to report infiltrations that exceeded NRC limits, they would want their infiltration rates to go down. With lower infiltration rates, nuclear medicine procedures would be safer for patients and produce more accurate scans for physicians.
Last year, Lucerno shared the results of its quality improvement study involving the Lara System with the NRC. The study concluded that providers can drastically reduce the occurrence of infiltrations with dedicated monitoring and feedback to technologists.
On January 28, 2020, NRC announced it is conducting an independent evaluation and reviewing the loophole that has exempted infiltrations from reporting requirement for 40 years.
