
ACT Gets Funding for Pancreatic Cancer Treatment Device

Medical device startup Advanced Chemotherapy Technologies (ACT) just got another financial boost in its efforts to develop a targeted drug delivery system to attack pancreatic cancer.
The Raleigh-based company said it has been awarded a Phase IIb Small Business Innovation Research grant, expected to total $4 million over two years, from the National Cancer Institute (NCI) within the National Institutes of Health (NIH). The grant will support development of the company's ACT-IOP-003 drug delivery system to treat pancreatic cancer that is difficult or impossible to remove surgically.
The ACT device, which is about the size of a quarter, is designed to be implanted in the pancreas. It uses iontophoresis, a technique that passes electric current through the chemotherapy drug gemcitabine and drives it directly into the cancer tumor. The goal is to shrink the tumor’s size so it can be removed.
“We are thrilled and encouraged that NCI has chosen to award us this highly competitive Phase IIb grant,” said William Daunch, Ph.D., ACT’s chief technology officer. “It demonstrates the NIH’s positive recognition of the work we completed in our earlier phases, as well as their continued confidence in our program to accelerate this potentially life-extending treatment to patients.”
Pancreatic cancer – rare but deadly
Pancreatic cancer is relatively rare, accounting for only about 3% of all cancers in the U.S., according to the American Cancer Society. But it is among the deadliest, with a five-year survival rate of only 10%. And since there currently is no effective screening, tumors often are too advanced when discovered to be surgically removed or effectively treated.
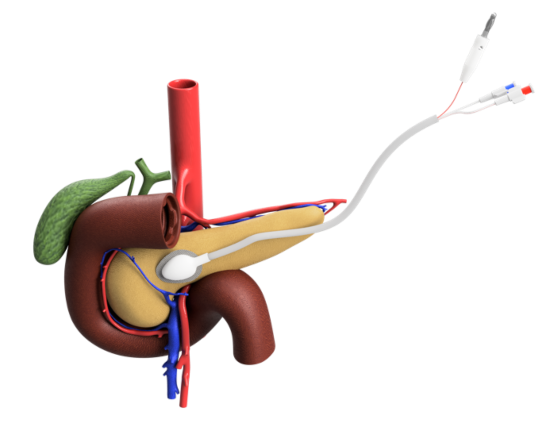
ACT said its drug delivery system has several advantages over traditional chemotherapy. It can deliver significantly higher concentrations of gemcitabine directly to the tumor, while limiting damage to surrounding healthy tissue. It can shrink tumors to a size that allows for removal, the only way to cure the disease. And by decreasing overall toxicity, it allows patients to better tolerate other conventional treatments.
The system was originally developed at the University of North Carolina at Chapel Hill in the labs of Jen Jen Yeh, a professor of surgery and oncology, and Joseph DeSimone, a former UNC chemistry professor who also is founder of another UNC spin-out, the 3-D printing startup Carbon. Some of the components of the ACT device are made with Carbon’s technology.
The NIH funding is just the most recent financial support ACT has received for the pancreatic cancer drug delivery device. Earlier this year, the company got $2.5 million in backing from Omaha-based investment firm Spectrum Financial. And in late 2020 it was awarded $5.5 million in a Series A round of financing led by Khosla Ventures, a Menlo Park, California-based venture capital fund.
Three years after the privately held company was formed in 2014, the North Carolina Biotechnology Center also provided it with a $250,000 Small Business Research loan to support efforts to advance the treatment to Phase I testing.
