
The Importance of Supporting Patient-Focused Innovation In NC

Necessity may not always be the mother of invention, but it helps.
Witness the growing number of North Carolina medical technology startups that have turned patient-focused innovations into viable businesses.
It all begins by recognizing an unmet need and then looking for ways to fill it. That was the case with Shawn Gage, a former physician associate in vascular surgery at Duke University Medical Center who founded InnAVasc Medical, Inc. He’s now the company’s director of clinical operations.
For close to 15 years, Gage treated thousands of patients with a variety of vascular disorders and complications. About half of them suffered from kidney disease or kidney failure and needed frequent dialysis to filter and cleanse their blood. As a result, many had synthetic tubes – called arteriovenous grafts – surgically implanted in their arms to connect a vein to an artery for better access to the blood stream.
The problem is that repeated needle sticks – or cannulation, in medical terms –may cause grafts to break down, bleed or collapse. And that can lead to serious complications.

So Gage and his Duke clinical partner and mentor – former Duke vascular surgeon Jeffrey Lawson, M.D., Ph.D. – went to work to find a better solution. They came up with a new kind of graft with two easily identifiable, protective pods specifically designed to make cannulation and blood stream access safer. The graft self-seals after cannulation and resists accidental needle punctures. The “sort of bullet-proof dialysis graph,” as Gage describes it, is simple yet effective.
With the help of Duke University’s Office of Licensing and Ventures, the two clinicians patented their design in the U.S. and Europe. InnAVasc Medical was founded in 2013.
The company has continued to raise funds to support its development efforts, including two loans from the North Carolina Biotechnology Center, a phase IIB Small Business Innovation Research (SBIR) grant from the National Institutes of Health, and a phase II SBIR grant from the National Science Foundation.
These allocations are currently being used to conduct a pivotal clinical study to gauge the safety and effectiveness of the graft Gage and Lawson designed.
They believe their innovation also has the potential to make the transition to home hemodialysis safer and less anxiety provoking so more patients can take advantage of the benefits of home therapy.
Surgeon’s Innovation Leads to Founding of Deep Blue
Another Duke physician, plastic surgeon Howard Levinson, helped create a mesh to prevent the reoccurrence of hernias following abdominal surgery.
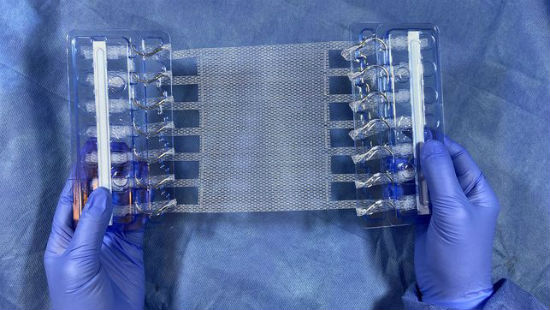
Deep Blue Medical Advances, Inc. – the company Levinson founded in 2015 – developed the product, called T-Line Hernia Mesh. It provides superior anchoring strength and eliminates a key point of failure for conventional mesh products – the mesh, suture, tissue interface. NCBiotech has supported Deep Blue’s development with two Small Business Research Loans.
Hernia repair failures are not an inconsequential problem. More than 400,000 abdominal hernia surgeries are performed each year in the United States at a cost of about $2.5 billion. And close to 30% of conventional repairs fail over the long term. Deep Blue hopes to improve that statistic.
In August 2020, the company announced it had received clearance from the U.S. Food and Drug Administration for T-Line with integrated suture-like extensions. The company currently is conducting a targeted clinical launch.
410 Medical’s LifeFlow delivers life-saving fluids during emergencies
Just down the road in Raleigh, WakeMed pediatric critical care physician Mark Piehl has developed a device to help inject fluids into patients quickly and easily in emergencies.

Dubbed the LifeFlow PLUS Blood and Fluid Delivery System, it’s a hand-held, intravenous administration set that rapidly delivers blood, blood components, and other resuscitative fluids directly to patients. The device is intended for those suffering from hemorrhagic shock or life-threatening injury. It can be used for both adult and pediatric patients.
That’s according to 410 Medical, the company Piehl formed in 2013 with venture capitalist Luke Roush. 410 Medical focuses on technology that can improve the resuscitation of the critically ill.
LifeFlow Plus enables providers to precisely titrate the volume of fluids to meet the specific needs of patients. Because it is approved for use with blood and fluids, it can address a broader set of patient conditions including septic shock and cardiac arrest, the company said.
The U.S. Food and Drug Administration cleared the first LifeFlow device in 2016. Since then, it has been used by a number of top health care systems including WakeMed, Cincinnati Children’s Hospital, and Children’s Hospital Colorado.
Perfusio’s technology measures blood flow during surgery
In Greenville, Bruce Ferguson, M.D. helped develop a non-invasive medical imaging technology that gives physicians instantaneous information about blood flow distribution during surgeries.

Ferguson, a retired cardiothoracic surgeon, served as inaugural chairman of the Department of Cardiovascular Sciences at East Carolina University’s School of Medicine. Now he’s chief medical officer of Perfusio Corp. (formerly RFPi, Inc.), the company created to develop and market the technology.
Certes, Perfusio’s first FDA-cleared Class II device, uses multiple wavelength illumination and artificial intelligence to visualize the actual blood flow distribution in tissues. This is key to avoiding surgical complications caused by inadequate blood flow that goes undetected in visible light.
Perfusio said the technology is based on laser speckle contrast imaging that displays real-time multi-spectral physiologic visualization (MSPV) of blood flow and perfusion – the rate at which blood is delivered to tissue. MSPV immediately identifies which tissues have normal flow and which do not, allowing the surgeon to find and correct problems at the time of the procedure. The information also is captured in Perfusio’s ARMUS Cloud Repository for future predictive analytics.
Certes is safe for patients and providers – even when used multiple times during a surgery, according to Perfusio. It doesn’t disrupt the procedure or require patient contact, dyes or ionizing radiation.
NCBiotech awarded the company a $250,000 Small Business Research loan in 2017 to help support the development of the MSPV platform.
“Dr. Ferguson is a perfect example of a clinician who has recognized a problem and created a solution,” said Greta Brunet, senior director of investments and co-leader of the Clinician Innovation Initiative at the North Carolina Biotechnology Center. “Health care providers on the front line are ideally positioned to identify challenges and play a role in developing meaningful solutions. We want to make sure they have all the support they need to take their ideas to the next level.”
Supporting clinician innovation in NC
Medical innovation continues to play an important role in North Carolina, for providers and patients alike. And Brunet thinks it can be even more of a game changer, if practitioners have the resources they need at their fingertips.
“Since most clinicians don’t receive innovation or entrepreneurship-related education during medical training, developing solutions to health care problems can be challenging,” she pointed out. “As a result, clinicians may drop their ideas before they get started. If we provide the support they need to navigate the creative process, from concept through execution, technologies that have the potential to transform health care may get the opportunity to do just that.”
With that goal in mind, NCBiotech and UNC FastTraCS are sponsoring a two-day virtual conference, Accelerating Health Care Innovation in North Carolina: Charting the Course. The project also has received support from NC IDEA, a private foundation whose vision is “to help North Carolinians achieve their entrepreneurial ambition to start and grow high potential companies.”
The objective of the event is to provide the insights and strategies current and future health care professionals need to turn good ideas into useful clinician innovations. It will be held September 23 and 24 from 11:30 a.m. to 3:30 p.m.
