
Deep Blue to Introduce New Hernia Mesh Product

The newest medical advance from Deep Blue Medial Advances has just received U.S. Food and Drug Administration marketing clearance.
The agency’s 510 (k) device authorization allows the Durham-based med tech startup to launch T-Line Hernia Mesh with added anchoring strength. The product, invented by surgeons, helps prevent the occurrence or reoccurrence of hernias following abdominal surgery. Deep Blue plans to introduce T-Line in the third quarter of this year.
The North Carolina Biotechnology Center awarded a $200,000 loan to the company in 2019 to support the FDA filing.
Abdominal pressure from activities like lifting or coughing can cause sutures to cut or pull through tissue or mesh in post-surgical patients. Deep Blue said T-Line eliminates a key point of failure for conventional mesh products – the mesh, suture, tissue interface. It increases anchoring strength and prevents mesh from failing in much the same way snowshoes keep you from sinking into snow – reducing stress by spreading force over a greater area.
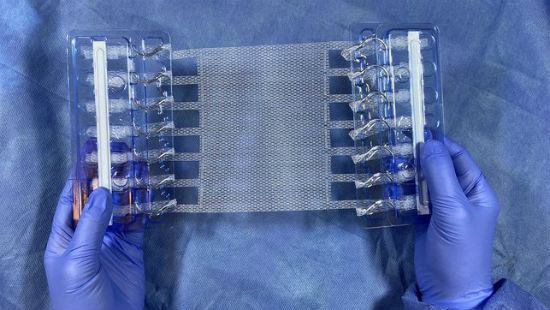
Hernia repair failures are nothing to be sneezed – or coughed – at. More than a million hernia surgeries are performed in the United States annually at a cost of about $2.5 billion. The fewer failures the better, both for patients and for healthcare spending. That’s where Deep Blue hopes to make a difference.
The long-term failure rate is 32% using conventional mesh and 63% using sutures alone, according to Deep Blue CEO Bill Perry. He said extensive lab and bench testing show that the T-Line Mesh has a 275% greater anchoring strength than the current standard of care.
“Sewing a bit of each extension into the abdominal wall, in lieu of traditional sutures, significantly increases mesh anchoring strength and thus the durability of repair,” added Howard Levinson, M.D., a Duke Health plastic surgeon who founded Deep Blue. “We believe this approach will greatly improve patient outcomes without necessitating significant changes to current surgical practice.”
Deep Blue got its start in 2014. In addition to T-Line Mesh, Levinson is working on other products that include an anti-biofouling Foley catheter, a non-invasive light imaging technology to diagnose skin disorders, and tissue-engineered skin that resists contraction.
