
Deep Blue Gets FDA Clearance to More Broadly Market its Hernia Mesh Product

Deep Blue Medical Advances has just gotten the go-ahead to expand the market for its unique T-Line Hernia Mesh product, which it commercially introduced in 2020.
The Durham-based company has received 501 (k) clearance from the U.S. Food and Drug Administration to sell T-Line Mesh for the sublay technique in open hernia surgery. Sublay procedures are the most widely performed open abdominal surgery for large-incision hernias.
Hernias occur when layers of the abdominal muscle become weak or tear, allowing internal organs or tissue to bulge under the skin.
Deep Blue said the OK from the FDA significantly expands the patient population that can be treated with T-Line. The product, invented by surgeons, helps prevent the occurrence or reoccurrence of hernias following abdominal surgery.
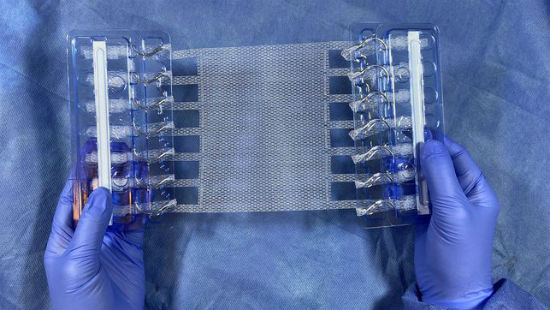
It eliminates a key point of failure for conventional mesh products – the mesh, suture, tissue interface. And it increases anchoring strength and prevents mesh from failing in much the same way snowshoes keep you from sinking in the snow – reducing stress by spreading force over a greater area.
T-Line’s design allows surgeons to make mesh tension adjustments and provides other potential surgical advantages compared to conventional mesh applications, according to the company.
“Many hernia surgeons prefer the sublay technique for hernia repair and have been eager to use T-Line for this,” said Howard Levinson, M.D., Deep Blue’s founder and chief medical officer.
“So we are thrilled to receive this clearance to enable broader clinical use.”
Hernia surgery is nothing to be coughed at. Four to five million abdominal incisions are performed in the United States each year, with hernias occurring after about 25% of these procedures, Deep Blue said.
Failure rates for long-term abdominal hernia repair are as high as 32% using conventional mesh products and 63% with suture repair only. The surgery is one of the five most common procedures performed in the U.S. by general surgeons, according to Deep Blue CEO Bill Perry. “This expanded clinical indication allows our growing number of clinical sites to address this important problem in significantly more patients with T-Line Mesh,” he added.
The company has estimated that the global market for hernia devices is about $1.1 billion.
Deep Blue was established in 2015 by Levinson, a plastic surgeon and researcher at Duke University. The North Carolina Biotechnology Center has strongly backed the company’s growth, providing a $75,000 Technology Enhancement Grant in 2016 to support Levinson’s hernia mesh work. NCBiotech followed up with a $250,000 loan in 2017 and a $200,000 Small Business Research Loan in 2019 to help gain FDA marketing clearance for T-Line.
The company also raised more than $7 million in venture capital earlier this year to commercialize its mesh device and to support related research.
In addition to T-Line Hernia Mesh, Deep Blue has a portfolio of other hernia and surgical products in development.
