
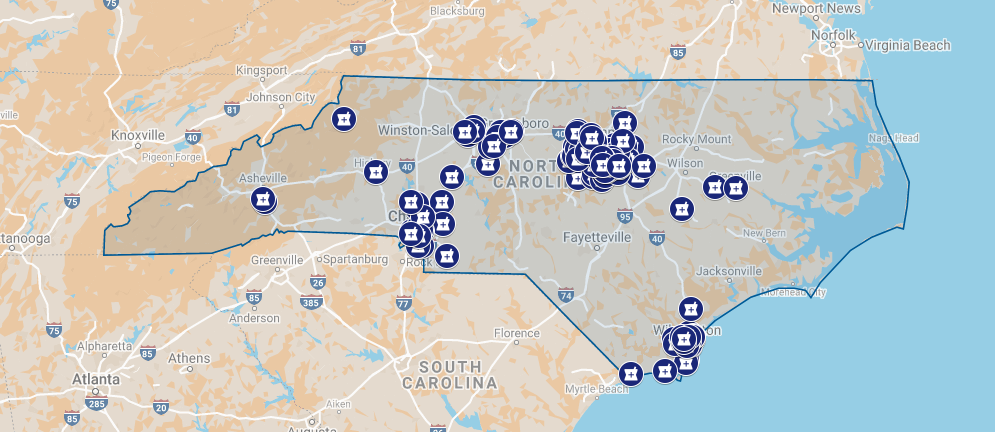
FUJIFILM Diosynth Biotechnologies, a global contract development and manufacturing organization (CDMO), plans to invest $1.2 billion to double the size of its manufacturing facility currently under construction in Holly Springs.
When completed, the site will be the largest end-to-end cell culture CDMO facility in North America, housing state-of-the-art biopharmaceutical manufacturing equipment and technology, including two large manufacturing suites for large-scale monoclonal antibody drug substance and drug product manufacturing.



























 ,
, 




