
URO-1 prepares for commercial launch of prostate biopsy platform
A Greensboro medical device company is poised to launch a biopsy platform that includes a needle with a proprietary and patented design that will offer physicians a more thorough, accurate and efficient tissue sampling and retrieval method.
Earlier this month, URO-1 Inc. was awarded U.S. Patent #11,903,569 for the design of its SUREcore needle for soft tissue biopsy. The device provides physicians with higher tissue volume, improved quality and consistency compared to traditional needles used for tissue biopsy, the company said. URO-1 intends to introduce its products initially for use in prostate biopsies and then expand into other areas of cancer diagnostics.
“I underwent prostate biopsy and was treated for prostate cancer at the University of California, San Francisco,” URO-1 CEO Ted Belleza said in an interview. “My urologist admitted that the instruments and methods used to take samples from the prostate have not changed in decades. More tissue means a higher probability of finding tumor cells. And better-quality tissues facilitate interpretation by a pathologist.”
URO-1 plans to launch a suite of products named SUREcore™ and coreCARE™ at the annual conference of the American Urological Association in early May and begin volume production of the devices this year. These milestones would follow a series of steps that began with the company’s launch in 2017 in Winston-Salem and bringing on Belleza, a medical device industry veteran, as CEO that same year.
Loan, financing provide springboard
In its early stages, URO-1 got a boost for its product development efforts from the North Carolina Biotechnology Center, which provided a $250,000 Small Business Research Loan in 2018.
URO-1 announced completion of its Series A financing round in early 2024. The $8 million round, led by Acorn Campus of Taiwan, also included funds from angel investors in Tennessee, California, Florida, and Kentucky.
About the same time, URO-1 received clearance for its SUREcore needle and coreCARE specimen retrieval kit from the U.S. Food & Drug Administration. The company has spent the past two years clinically validating the performance and utility of SUREcore and coreCARE while preparing to raise additional capital and build a production facility in Greensboro.
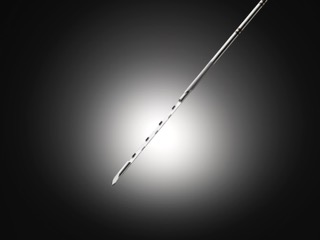
“The SUREcore needle provides a larger volume of prostate tissue compared with the standard of care needle, which could result in a better chance of diagnosing any potential cancer that is present,” Jeffrey Proctor, M.D., of Georgia Urology in Marietta, Ga., said in a statement. Dr. Proctor has used the device in more than 90 procedures.
“The amount of prostate tissue obtained on conventional needle biopsy can be extremely variable, sometimes inadequately sampling the prostate gland which results in suboptimal representation of the prostate cancer,” said Jonathan Epstein, M.D., an expert in urological pathology. “The SUREcore needle is a novel design that consistently and reproducibly obtains uniform longer and more intact cores of prostate tissue, which is a major advantage over current conventional needle biopsy methods.”
Next steps
While planning for the May 2024 launch, URO-1 is gearing up for its presentation at the Venture Connect 2024 conference in Raleigh in March. Belleza expects the presentation to boost efforts to raise the additional capital needed to scale production.
Full-scale manufacturing of the device will place URO-1 squarely in the U.S. market for prostate biopsy instruments, valued between $200 million and $300 million per year, Belleza said. Of the roughly 3 million soft tissue diagnostic biopsies performed annually in the United States, about half are conducted in the prostate for diagnosing and monitoring prostate cancer, the most common cancer and second-leading cause of cancer-related deaths in men in the U.S.
Kathy Meserve, senior director of investments at NCBiotech, said URO-1’s latest milestones validate the company’s sequence of raising capital, testing the product, clearing regulatory steps, and establishing agreements before moving to full production and additional fundraising.
“It’s a textbook example of taking an idea and methodically moving through all the steps to introduce a useful, viable product,” she said. “It’s going to be exciting to see how URO-1 changes the way biopsies are conducted and improves patient care as a result.”
