
Humacyte Begins Phase 2 Study of Bioengineered Vessel for Vascular Trauma

Humacyte, a Durham-based regenerative medicine company, has begun a Phase 2 clinical trial of Humacyl, its bioengineered blood vessel, in patients with vascular trauma.
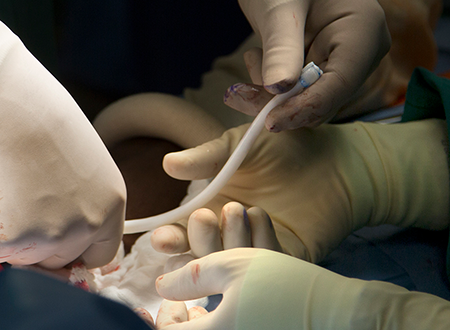
The study will evaluate Humacyl for vascular replacement or reconstruction in about 40 adult patients with life- or limb-threatening vascular trauma who require surgical repair, the company said in a news release. The trial is being conducted at six sites in the United States, and enrollment will continue for 24 months.
“We believe we have a unique and compelling solution to reduce the significant loss of life related to traumatic vascular injuries of civilian victims of violent crimes, automobile accidents, industrial incidents and more,” said Jeffrey Lawson, M.D., Ph.D., president and chief executive officer of Humacyte. “Humacyl has the potential to be a first-in-class therapy for long-term blood vessel restoration or reconstruction of traumatic vascular injuries, and the advancement of our Phase 2 study represents a significant milestone to investigate the potential of Humacyl in clinical vascular trauma.”
Initial patients were enrolled at Rutgers University Hospital in Newark, N.J., and the R. Adams Cowley Shock Trauma Center in Baltimore. Other sites expected to enroll patients include Johns Hopkins University in Baltimore, Marcus Trauma Center in Atlanta, Ryder Trauma Center in Miami and Rocky Mountain Regional Trauma Center in Denver.
“A tremendous medical need exists for patients with traumatic injuries who are in need of vascular reconstruction both in the civilian and military populations,” said Michael Curi, M.D., MPA, chief of the division of vascular surgery at Rutgers. “Advancements in tissue engineering, such as Humacyte’s bioengineered vessel, have the potential to be a game-changer for both patients and the brave members of our armed forces who may experience this critical need. Rutgers University is thrilled to participate in this significant investigational Phase 2 vascular trauma clinical study as part of our ongoing commitment to improve the lives of every patient, every day.”
Thomas Scalea, M.D., Francis X. Kelly distinguished professor of trauma and physician-in-chief at the R. Adams Cowley Shock Trauma Center at the University of Maryland, said, “We are constantly exploring new and innovative approaches to treating life-threatening injuries. We look forward to evaluating this bioengineered blood vessel in the treatment of appropriate patients requiring vascular surgical repair.”
Humacyte said it expected topline patient data from the study to be available in late 2020.
Humacyl is also currently in a Phase 3 pivotal trial in the U.S. and Europe as a conduit for hemodialysis in patients with end-stage renal disease. The company plans to seek regulatory approval in both regions upon completion of the trial.
Humacyte is also conducting a Phase 2 trial of Humacyl as a bypass graft in patients with peripheral arterial disease.
Company well financed

president and CEO
Humacyte is a privately held company founded by Laura Niklason, M.D., Ph.D., in 2004. The North Carolina Biotechnology Center awarded Humacyte a $150,000 Small Business Research Loan in 2006.
In 2015 the company raised $150 million in a Series B preferred stock financing ̶ among the largest ever by a life science company in North Carolina.
In March of this year Humacyte raised $75 million in a Series C preferred stock financing, led by a consortium of private investors and new investors.
In June, Humacyte formed a global partnership with Fresenius Medical Care, the world's largest provider of dialysis products and services. It received a $150 million equity investment as part of the deal, while Fresenius obtained exclusive global rights to commercialize Humacyl and took a 19 percent ownership stake in Humacyte.
Earlier this year Humacyte completed construction of a new, state-of-the-art research and development and bioprocessing facility in Durham.
Novel technology yields off-the-shelf product potential
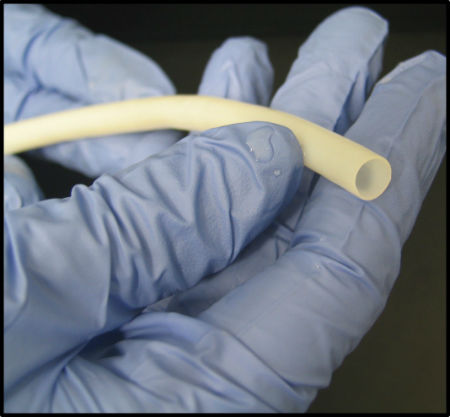
Unlike most regenerative medicine companies, Humacyte does not directly use a patient’s own cells or tissues as therapies or products. Instead, it uses a proprietary platform technology to engineer human, extracellular matrix-based tissues that can be shaped into tubes, sheets or particulate conformations, with properties similar to native tissues.
Humacyl is derived from banked human vascular cells that are seeded onto a degradable scaffold and then cultured so they secrete a matrix that forms a tissue in the shape of the scaffold. The vascular cells are then removed to avoid triggering an immune response or infection in patients receiving the tissue.
The resulting vessels, known as human acellular vessels, could be used in hospitals as readily available, off-the-shelf products when needed. They are intended to improve patient efficacy, safety and treatment outcomes.
