
Durham’s Baebies to Market Fast COVID-19 Test in Europe

Baebies Inc., a medical device and diagnostics company in Durham, has received regulatory clearance to commercialize a test for the COVID-19 virus in Europe.
The company can begin marketing its FINDER SARS-CoV-2 test across the European Union because it received a CE Mark, a designation confirming that the test is safe and effective.
“CE Mark is an important regulatory milestone for Baebies to expand the reach of our FINDER SARS-CoV-2 test and introduce the power of digital microfluidics to new markets across the world,” said Richard West, co‑founder and chief executive officer of Baebies. “Our technology unlocks access to rapid, accurate testing at the point of care so communities can manage the threat of COVID-19 now and into the future.”
Leveraging digital microfluidics
Baebies’ testing platform uses digital microfluidics technology to streamline and miniaturize the conventional RT-PCR process, enabling lab-quality results at the point of care.
The test detects the SARS-CoV-2 virus in nasopharyngeal and nasal swab specimens in 17 minutes or less. Conventional RT-PCR testing results are typically reported after 24 hours.
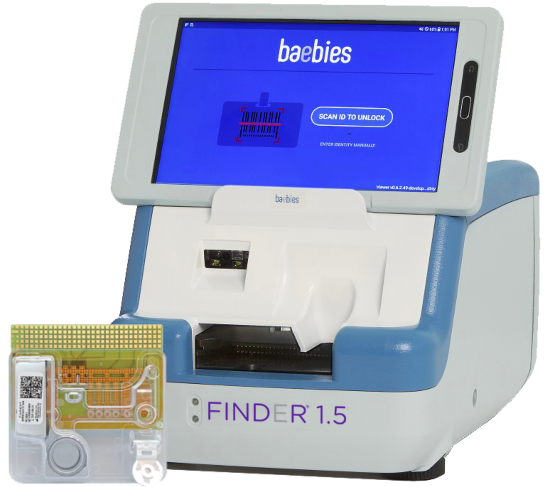
The testing platform features a compact, toaster-sized instrument with a mini tablet for user interface. Each test is run on a single-use disposable cartridge.
The FINDER 1.5 Instrument helps laboratories, hospitals and other point-of-care settings meet the demand for fast and accurate testing. It delivers the speed of a rapid antigen test and the accuracy of an RT-PCR lab test.
RT-PCR is an abbreviation for reverse transcription polymerase chain reaction. It is a molecular test that amplifies and detects the COVID-19 virus’s genetic material.
RT-PCR is considered the gold standard for COVID-19 tests due to its high accuracy.
Although COVID-19 restrictions are being lifted, testing remains an important tool to reduce the spread of the virus.
Company Focused on Early Detection
Baebies is a growth-stage company developing products to enable early detection of diseases and syndromes in children and adults.
Guided by the vision that “everyone deserves a healthy start," the company delivers millions of tests globally every year to central laboratories and point-of-care sites around the world.
Baebies is a pioneer in digital microfluidics, which maximizes diagnostic yield from low-volume samples. Its technology has won multiple awards, including the Disruptive Technology Award in 2020 from the American Association for Clinical Chemistry in the organization’s “search for the next innovative testing solution that will transform patient care.”
The company received a $500,000 Strategic Growth Loan from the North Carolina Biotechnology Center in 2014, when it was founded. The support helped it raise $13 million in equity financing the following year.
