
Aerie Begins Clinical Trial of Implantable Eye Drug

Durham-based Aerie Pharmaceuticals, a developer of therapies for eye diseases, has begun patient dosing in a first-in-human clinical trial of an implantable drug for treating two retinal diseases: neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME).
The trial will evaluate the safety and preliminary efficacy of AR-13503 SR Implant, a bio-erodible polyesteramide polymer implant that provides controlled release of the drug AR-13503 over a sustained period. AR-13503 is a proprietary, small-molecule inhibitor of both Rho kinase and Protein kinase C that is designed to be administered about once every six months by injection into the eye.
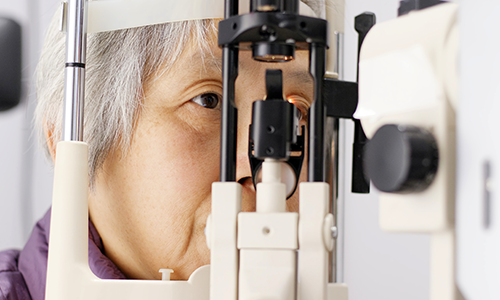
Preclinical studies suggest that AR-13503 may inhibit angiogenesis, or the formation of new blood vessels; preserve the blood-retinal barrier; and reduce retinal fibrosis in retinal diseases, while potentially reducing the treatment burden associated with more frequent eye injections.
The new clinical trial will evaluate AR-13503 alone and in combination with aflibercept, a drug marketed as EYLEA by Regeneron Pharmaceuticals. Aflibercept is a vascular endothelial growth factor (VEGF) inhibitor approved for the treatment of retinal diseases.

shows how small it is.
“We are excited to be initiating human clinical evaluation of a novel treatment pathway for common, sight-threatening retinal diseases,” said Vicente Anido Jr., Ph.D., chairman and chief executive officer of Aerie. “Because AR-13503 may address several distinct components of the disease process in nAMD and DME, we believe the AR-13503 SR Implant has the potential to be an important addition to the treatment armamentarium in retina, particularly by helping expand the options for individualizing therapy.”
Anido said the study is designed to confirm pre-clinical observations that AR-13503 could be effective alone or in tandem with VEGF inhibitors, and to help select an appropriate dose for later stage trials.
“It will also provide the first clinical data for our polyesteramide bio-erodible polymer implant technology, which allows us to deliver small-molecule drugs to the back of the eye while extending treatment duration,” he said.

The multi-arm, 24-week study will be conducted in two stages. The first phase is a multicenter, open label, dose-escalation study of the safety and tolerability of a single injection of AR-13503 SR Implant, using two doses, in up to 12 patients. The second phase, enrolling up to 90 patients, will be a multicenter, single-masked, randomized, parallel group study of the safety and preliminary efficacy of low- or high-dose AR-13503 SR Implant, dosed alone and in combination with aflibercept.
More information about the study is available at www.clinicaltrials.gov under the study designation NCT03835884.
Retinal diseases a $6 billion market
The retina is a thin membrane lining the back of the eye composed of highly specialized cells that convert visible light into electrical impulses that reach the brain through the optic nerve.
A variety of diseases can damage the retina and cause visual impairment or permanent blindness. They include progressive disorders such as age-related macular degeneration (AMD) and diabetic macular edema (the macula is the central portion of the retina).
In the United States, AMD is the leading cause of significant loss of visual clarity in people over age 50, and diabetic retinopathy is the most common cause of irreversible blindness among working-age people. As a result, the retinal disease market is the largest therapeutic category in ophthalmology, with revenues in the United States reaching about $6 billion in 2018.
Because current medical therapies are not effective for many patients and don’t stop disease progression, new treatment options are needed, the company said.
Products on the market
Aerie is focused on the discovery, development and commercialization of first-in-class therapies for the treatment of open-angle glaucoma, retinal diseases and other diseases of the eye.

Its first product, Rhopressa (netarsudil ophthalmic solution), was launched in the United States in April 2018 as a once-daily eye drop for reducing elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. A second product for reducing IOP, Rocklatan (netarsudil and latanoprost ophthalmic solution), was launched earlier this year.
Aerie is developing additional product candidates and technologies in ophthalmology, including for wet age-related macular degeneration and diabetic macular edema.
The company is a spinout of Duke University based on the glaucoma research of the late David L. Epstein, M.D., a clinician and scientist who chaired the Department of Ophthalmology at Duke’s School of Medicine for 22 years.
Aerie has about 380 employees at its sites in Durham, Irvine, Calif., Bedminster, N.J., Tokyo, Henley-on-Thames, England, and Dublin, and in its U.S. field sales force. It has also completed construction of a manufacturing plant in Athlone, Ireland, that is scheduled to come online in 2020.
The Durham headquarters has 80 employees and is adding more.
Aerie became a publicly traded company in 2013, and its stock shares are traded on the NASDAQ Global Market stock exchange under the symbol AERI.
