
410 Medical Completes $7.5 Million Funding Round
Durham-based 410 Medical, a medical device company developing new technologies for emergency and critical care, has completed its $7.5 million Series B venture capital financing.
The funding, which had an initial closing late in 2022, was led by Durham-based Hatteras Venture Partners with participation from existing investors including Orlando Health Ventures, OSF Healthcare and Raleigh-based WakeMed, and new investors Ballad Ventures and Catalyst by Wellstar.
The financing will be used to further develop and deploy 410 Medical’s lead product, LifeFlow, a hand-held device with a trigger for quickly and precisely infusing blood and intravenous fluids in critically ill patients.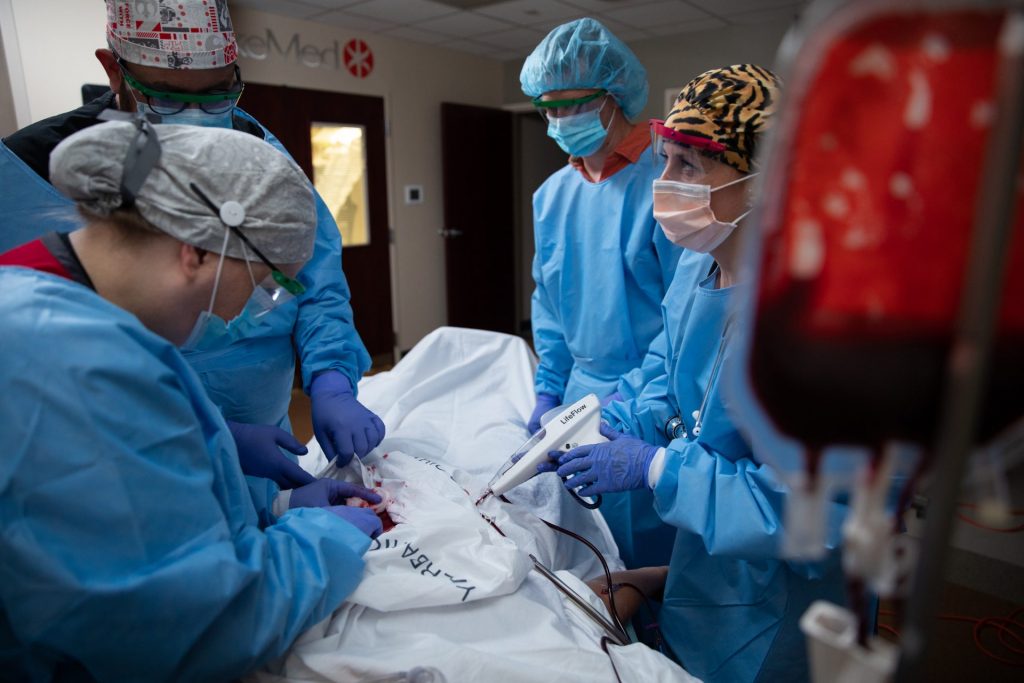
“Many years ago I was struck by our inability to provide timely resuscitation for patients with shock,” said Raleigh physician Mark Piehl, M.D., co-founder and chief medical officer at 410 Medical. “Now, 10 years since the creation of 410 Medical, emergency and critical care clinicians have used LifeFlow to improve care for over 20,000 patients. We are thankful for the many EMS, hospital and military partnerships we have developed and for the investors who have made this journey possible and helped us to affect patient care in the way that we have.”
Severe sepsis affects over 1 million adults and children each year in the United States and is the most frequent cause of death in hospitalized patients. Trauma is the leading cause of death in patients less than 45 years old.
In these and other conditions leading to hypotension and shock, rapid delivery of blood or IV fluid can be a life-saving therapy. With traditional infusion methods, however, clinicians often fail to achieve timely resuscitation or adhere to published guidelines, according to 410 Medical.
Since its clearance by the U.S. Food and Drug Administration in 2016, LifeFlow has been used at many healthcare systems including Orlando Health, Ballad Health and Wellstar. These three systems have invested in 410 Medical through their venture capital arms.
“We are enthusiastic about this partnership’s transformative impact on our communities, as we continue our unwavering pursuit of providing exceptional care to those we serve,” said Amit Vashist, M.D., chief clinical officer for Ballad Health and clinical advisor for Ballad Ventures, both based in Johnson City, Tenn. “Incorporating this leading-edge medical device into our pediatric and emergency settings has already made a significant impact in our ability to deliver timely and effective resuscitation to patients in need, elevating their chances of survival and promoting their overall well-being.”
Early funding from NC Biotech
410 Medical was founded by Piehl and venture capitalist Luke Roush in 2013.
In 2016 the company was awarded a $250,000 Small Business Research Loan by the North Carolina Biotechnology Center. The loan program supports business inception and research critical to developing products, processes or tools with clear commercial potential.
410 Medical raised $3.2 million in Series A venture capital in 2018 to commercialize LifeFlow. North Carolina investors in the funding round included WakeMed Health & Hospitals and the North Carolina Venture Capital Multiplier Fund, managed by Hatteras Venture Partners.
The company lists 21 employees on its website.
410 Medical’s name is derived from the calculation of a fluid-delivery goal in treating pediatric septic shock.
