
BPD Cell and Gene Therapy Symposium & Vendor Show

There are more than 35 Cell and Gene Therapy (CGT) companies and CDMO operations in North Carolina, and new companies are considering locating manufacturing sites here. Join us on April 25 for the Biomanufacturing and Process Development (BPD*) group’s 5th annual CGT Symposium and Vendor Show. This a full-day event where you can learn about CGT biomanufacturing progress and challenges via engaging presentations. Come to network and make new contacts.
This event will feature more than 35 sponsors and vendors with displays on current up and downstream processing equipment, as well as analytical instruments and assays to support your CGT business and career.
Agenda
8:00 - 9:00 a.m. | Attendee Check In/Registration
9:00 - 9:05 a.m. | Opening of Symposium, Ronna E. Dornsife, MS (AskBio) Symposium Co-Chair
Welcome by Platinum Sponsor: Kymanox, Stephen M. Perry, BS, CEO & Founder
9:05 - 9:10 a.m. | Introduction of speakers, Justin Watkins, PhD (KBI Biopharma) Symposium Co-Chair
9:10 - 10:20 a.m. | Speaker Session I
Ben Josey, PhD, Field Application Scientist, Corning Life Sciences
“Interferometric Light Microscopy for Rapid Virus Titering and Characterization of Nanoparticle Preparations: Applications in Downstream Process Development and Sample Normalization”
Peter Holper, BS, Sr. Application Scientist, Biopharma SCIEX
“From Raw Material to Drug Product: Analysis of Therapeutic Gene Therapy Products”
10:20 - 11:00 a.m. | Morning Break, Visit Sponsor and Vendor Tables, and enjoy Networking
11:00 - 11:05 a.m. | Introduction of Speakers, Justin Watkins, PhD (KBI Biopharma) Symposium Co-Chair
11:05 - 12:15 a.m. | Speaker Session II
Maria Oyaski, MS, MA, RAC (US, EU), Regulatory Consultant
“What’s New, and What’s Next, with FDA’s Current Thinking for Cell and Gene Therapies”
Carmen Amador, BA, Chief Quality Officer, LaSalle Group and Jose Vidal, PhD, CEO, CytoImmune Therapeutics
“Proven Roadmap to Achieve Successful BLA”
12:15 - 1:45 p.m. | Lunch Break, Visit Sponsor and Vendor Tables, and enjoy Networking
1:45 - 1:55 p.m. | Welcome by Platinum Sponsor: Flow Sciences Inc., Allan Goodman, PhD, Regional Sales Manager. Speaker Introductions, Larissa Benavente, PhD, (AskBio) BPD Steering Committee Member
Introduction of Speakers, Ronna E. Dornsife, MS (AskBio) Symposium Co-Chair
1:55 - 3:05 p.m. | Speaker Session III
Greg Swan, Business Development, Avantor Sciences
“Demonstration of a critical quality framework for chemical lysis agents through the validation of a novel cell lysis solution that improves vector yield”
Robin Newman, BS, CQV Lead/Project Manager, Core Services Group
“Breakthroughs in Therapeutic Protein Production and Drug Delivery: Defying the Norms of Process Design with Eco-Friendly Reagents and Novel Technologies”
3:05 - 3:45 p.m. | Afternoon Break, Visit Sponsor and Vendor Tables, and enjoy Networking
3:45 - 3:50 p.m. | Introduction of Speakers, Larissa Benavente, PhD, (AskBio) BPD Steering Committee Member
3:50 - 5:00 p.m. | Speaker Session IV
Andreas Solomos, PhD, Director Virology Operations, CGT, WuXi Advance Therapies
“Cell and Gene Therapy Regulatory Testing; Supporting Products from Development to Market”
Jeff Odum, BS, MS, CPIP, Practice Leader, ATMPS & Biologics, Genesis Engineers
“The Journey to ATMP Commercialization”
5:00 - 5:05 p.m. | Thank you to Organizers, Sponsors, Vendors, and Attendees
Platinum Level Sponsors

Gold Level Sponsors

![]()




Silver Level Sponsors






![]()

![]()


Participating Vendors
Actylis, AirClean Systems, Akron Bio, Aldevron, Biologos, BA Sciences, Chemglass Life Sciences, Contec, Corning, Cygnus Technologies, Gyros Protein Technologies, LabRepCo.com, Miltenyi Biotec, NanoEntek, New England Biolabs, Parker Hannifin, Proteintech Group, SCIEX, Solvias, STERIS, Unchained Labs, Waters Corporation
Directions/Parking
The Friday Center is located three miles east of UNC-CH campus, off Highway 54 East (Raleigh Road). From Raleigh or RDU Airport, take Interstate 40 Exit 273A. From Greensboro or Durham, take I-40 Exit 273. Free parking is available for all attendees.
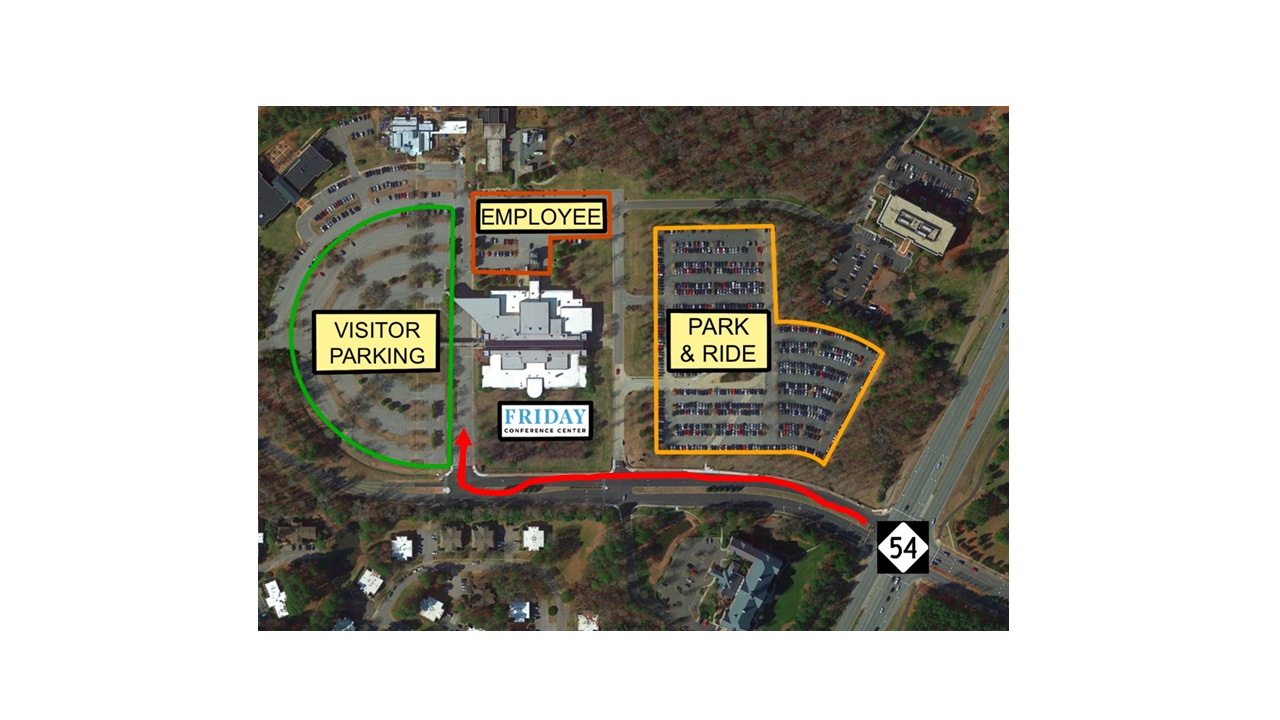
Registration
Free parking, Wi-Fi, lunch and refreshments are included with symposium registration.
Sponsor Information
Platinum Sponsors

Flow Sciences specializes in cutting-edge containment solutions for various industries, including pharmaceuticals, biotechnology, food, government and research applications. Our products, such as containment enclosures and fume hoods, ensure personnel safety and experiment integrity.
Our containment enclosures provide superior protection against hazardous substances, facilitating secure handling of potent compounds and volatile chemicals. With customizable features and advanced airflow control, they optimize workflow efficiency while maintaining strict containment protocols.
Similarly, our fume hoods effectively capture and remove harmful fumes, vapors, and particulates, safeguarding laboratory personnel from exposure to hazardous substances. Equipped with state-of-the-art airflow management systems and ergonomic design, they promote user comfort and operational efficiency.
Quality and safety are paramount at Flow Sciences. Our products undergo rigorous testing and certification to meet industry standards and regulatory requirements, ensuring customer peace of mind. With a focus on innovation and customer satisfaction, Flow Sciences is your trusted partner for advanced containment solutions in the laboratory technology landscape.

Kymanox has proven, collaborative, end-to-end solutions that help bring life science products to the market – and keep them there. We are a 300-person global professional services organization that supports modern medicine development through the integration of (i) Science, (ii) Engineering, (iii) Compliance (e.g., Quality, Regulatory), and (iv) Technical Project Management. Our work across Large and Small Molecules, Advanced Therapies, Medical Devices, and Combination Products (e.g., biologics in auto-injectors) provides us a wholly unique perspective in the industry that we leverage for our clients’ programs. Now in our 20th year of operation, we have a strong pedigree of supporting Cell & Gene Therapy (C>) innovation, including our work with the first-ever, FDA-approved cell therapy (i.e., Provenge), and supporting one of the first-ever gene therapy companies in the United States (i.e., Lentigen). With a diverse team of experts, Kymanox helps our clients navigate commercialization challenges that arise throughout a product’s life cycle – from early development to post-market – with optimized safety, quality, efficacy, and accessibility. The company strives to advance Life Science innovation through insightful solutions and collaboration, including leveraging the Kymanox Hyper-Virtual Model for both speed and de-risking. Our corporate vision and why is simply ”…because patients deserve better.” Kymanox was founded in 2004 and is headquartered in Research Triangle Park, North Carolina USA. We are backed by WestView Capital Partners, a Boston-based growth equity firm. For more information, visit https://www.kymanox.com/.
Gold Sponsors

Akuratemp LLC is a leading North Carolina-based company providing temperature-controlled packaging solutions for healthcare with advanced phase change materials for transporting and storing diagnostic samples, blood products, organs, cell cultures, stem cells, antibodies, proteins, and pharmaceuticals.
As a trusted supplier for the top 20 biopharmaceutical companies, the expertise and capabilities Avantor® offers are focused on solving the complexities of the evolving landscape within cell and gene therapy.
Avantor® can help you maintain the integrity and activity of cell and gene therapy products during upstream and downstream purification through the final fill. Avantor® has a team of local specialists to support a broad product portfolio of materials including:
-Cell culture components
-Fermentation media and supplements
-Growth factors
-Reagents and excipients
-Protein purification systems
-Single-use use products for fluid transfer that supports “closed-system” cell culture
-We carry a comprehensive range of products from brands you trust for gene therapy to genomics applications.

Together, we elevate science to the next level of possibility. Cell and gene therapies are set to become the new standard of health care but remain variable, complex, and difficult to manufacture with consistency.

The Eppendorf bioprocess portfolio offers comprehensive and scalable hardware and software solutions for R&D, process development, pilot and production. Our portfolio features upstream bioprocess solutions with working volumes from 50mL-2400L. We support a wide range of process types, including mammalian and microbial cultures, in glass, stainless steel, and single-use reactors.

MilliporeSigma is over 350 years old with a presence in over 66 countries. We are a Vibrant Life Science supplier of over 300,000 products, systems and services to the pharma and biotech industry. We have dedicated Emerging Biotech and Commercial Support teams to help empower our customers to solve their toughest challenges. By partnering with you from discovery and development through manufacturing and commercialization we can provide access to comprehensive services, process development tools and education programs and training. We are here to build partnerships and strengthen the biotech community. We help you get it right the first time: We do it for you, with you, or help you do it yourself.

As the advanced therapies business unit of WuXi AppTec, WuXi Advanced Therapies is a Contract Testing, Development and Manufacturing Organization (CTDMO) that offers advanced platforms and end-to-end solutions that enable the discovery, development, testing, manufacturing, and commercialization of cell and gene therapies. Our services and solutions accelerate time to market and support customer programs around the world. For more information, visit www.advancedtherapies.com.
Silver Sponsors

ACROBiosystems Group, founded in 2010 and listed in 2021, is a biotechnology company aimed at being a cornerstone of the global biopharmaceutical and health industries by providing products and business models innovation. The company spans the globe and maintains offices, R&D centers, and production bases in 12 different cities within the United States, Switzerland, England and Germany. ACROBiosystems Group has established numerous long-term and stable partnerships with the world’s top pharmaceutical enterprises, including Pfizer, Novartis, and Johnson & Johnson, and numerous well-known academic institutes. The company comprises of several subsidiaries such as ACROBiosystems, bioSeedin, Condense Capital, and ACRODiagnostics.
ACROBiosystems’ brands include FLAG, Star Staining, ViruStop, Aneuro, ComboX, GENPower, and many others. Its main products and services are recombinant proteins, kits, antibodies, scientific services, and other related products. ACROBiosystems employs a strict quality control system for its products that are used in biopharmaceutical research and development, production, and clinical application. This includes targeted discovery and validation, candidate drug screening/optimization, CMC development and pilot production, preclinical research, clinical trials, commercial production, and clinical application of companion diagnostics.
Through the continuous development of new technologies and products, ACROBiosystems Group creates value for the global pharmaceutical industry and actively empowers our partners. The company is dedicated to accelerating the drug development process, including targeted therapies, immunotherapeutic drugs, and its clinical applications, and contributes to global health.

Advanced Medicine Partners delivers what patients deserve and what regulators expect.
We provide process development, manufacturing, analytical development and testing for advanced medicines, specializing in viral vectors for gene and cell therapies. Our proprietary AAV manufacturing process minimizes impurities and generates industry-leading functional full capsid ratios. We bring extensive operational experience and have manufactured 350+ non-GMP batches and supplied 20+ preclinical studies, including IND-enabling efficacy and GLP toxicology.
After decades at biotech companies, we understand what it’s like to depend on a partner to deliver. Let us be your extended workbench!

StemFit™ is a pluripotent stem cell and regenerative medicine brand of Ajinomoto, with a product portfolio containing GMP-grade all animal origin-free iPSC/ESC culture media, MSC culture media, and growth factors. StemFit products meet the standards of GMP guidelines, USP 1043 ancillary material requirements to make successful a stable and robust clinical cell production. StemFit products are the first media and growth factors to be recognized by the Pharmaceutical and Medical Devices Agency (PMDA) in Japan as free of animal-origin components, free from viral contamination and suitable for GMP production.
Asklepios BioPharmaceutical, Inc. (AskBio), a wholly owned and independently operated subsidiary of Bayer AG acquired in 2020, is a fully integrated gene therapy company dedicated to developing life-saving medicines that have the potential to cure genetic diseases. The company maintains a portfolio of clinical programs across a range of neuromuscular, central nervous system, cardiovascular and metabolic disease indications with a clinical-stage pipeline that includes therapeutics for Pompe disease, Parkinson’s disease and congestive heart failure, as well as out-licensed clinical indications for hemophilia and Duchenne muscular dystrophy. AskBio’s gene therapy platform includes Pro10™, an industry-leading proprietary cell line manufacturing process, and an extensive AAV capsid and promoter library. With global headquarters in Research Triangle Park, North Carolina, and European headquarters in Edinburgh, UK, the company has generated hundreds of proprietary third-generation AAV capsids and promoters, several of which have entered clinical testing. Founded in 2001 and an early innovator in the gene therapy field, the company holds more than 750 patents and applications in areas such as AAV production and chimeric and self-complementary capsids. Learn more at www.askbio.com or follow us on LinkedIn.

Life sciences. Research. Precision manufacturing. If your life’s calling is in any of these or related fields, you need to know Beckman Coulter Life Sciences. Our mission is to empower those seeking answers to life’s important scientific and healthcare questions. Since 1935, the Beckman name has been synonymous with technologies that simplify and automate complex biomedical testing. Decades later, our global organization also came to embody the scientific legacy of the Coulter name. Today, Beckman Coulter Life Sciences is a trusted, worldwide resource for tools to help optimize research and manufacturing efficiency. Centrifuges. Particle counters/analyzers. Automated liquid handlers. Flow cytometers. Genomic reagents. All these products—and many more—continue to make a difference in people’s lives by improving the productivity of dedicated scientists, quality control experts and others. Wherever people need answers, from prestigious universities and major pharmaceutical companies to small biotech startups, food/beverage and electronics manufacturing facilities, you can find Beckman Coulter Life Sciences. For more details, visit beckman.com.

Caron Products is your trusted partner in providing cutting-edge laboratory automation solutions. With over 38 years in the industry, we specialize in designing and manufacturing high-quality equipment tailored to meet the specific needs of purchasers in research and industrial laboratories.
Our extensive product range includes state-of-the-art environmental chambers, CO2 incubators, stability storage systems, and operator protection solutions. Each product is meticulously engineered to ensure precision, reliability, and compliance with international standards.
At Caron, we understand the importance of creating controlled environments for scientific
experiments, sample storage, and automation processes. Our commitment to excellence extends beyond product design, encompassing dedicated customer support, customization options, and a focus on sustainability.
Discover how Caron Products can enhance your laboratory operations. Visit our website at
https://caronproducts.com/ to explore our comprehensive range of products.

With a sole focus on the biotechnology and pharmaceutical industry, ClinLab Staffing, a division of Focus Search Group, LLC., recruits specialized experts to support a wide range of academic and industrial environments. From individual placements to large projects, we ensure that every scientific initiative is safe, timely, successful, and profitable.

KACTUS is a biotech company that specializes in providing high-purity, high-activity, recombinant protein and enzyme products for a range of fields. We have more than 3000+ protein catalog products, many of which are hot targets in developing T-cell engagers. Our pMHC complex series is our best sellers series for people who work on TCR-T cell therapeutic development. In addition, we provide both research-grade and GMP-grade gene editing enzymes such as Cas9, Cas12a, MaxNulceases to support CGT research and large-scale manufacturing. Our product and service is a good match with the topic of this symposium and therefore, we would like to take this opportunity to participate in as a sponsor and get to know more people and seek more business opportunities.

MedChemExpress is a leading supplier of bioactive molecules including bioactive chemical compounds, peptides, recombinant proteins, and inhibitory antibodies. We provide a comprehensive list of fully customizable screening libraries including various bioactive compound libraries and fragment libraries. We also provide custom synthesis services and CMC/CDMO services.

Since 1984, the North Carolina Biotechnology Center has led life sciences technology-based economic development for the state by supporting the progression of ideas from the research lab to the marketplace. A private, non-profit state-funded corporation, NCBiotech invests in technology development through grants, in company development through loans, and in economic development through partnership development grants. Our transformational programs and activities develop strengths that yield high-paying life sciences jobs statewide. NCBiotech is headquartered in Research Triangle Park, with regional offices in Asheville, Charlotte, Greenville, Wilmington and Winston-Salem. To learn more, visit ncbiotech.org.

OrganaBio was founded in 2018 with the mission to become the hub for tissue sourcing, cell isolation, clinical sample processing and contract manufacturing services to support cell and gene therapy developers around the globe. At our core, we continually apply a data-driven approach with meaningful insights across our strategically located facilities to provide solutions to our clients where they need it most. Headquartered in Miami, Florida, OrganaBio delivers products and services that span the full development lifecycle – from proprietary tissue supply chains and cellular starting materials to expert development, testing, and other support services that expedite the path to clinical translation. Visit https://www.organabio.com for more information.

Refeyn pioneers a new generation of analytical instrumentation for rapidly measuring the mass of single molecules, viruses and other particles. In cell and gene therapy, mass photometry technology is used in vector characterization. From accurately quantifying empty/full ratios of AAV capsids in just minutes, to assessing the purity and stability of LVV and AdV, mass photometry detects the light scattered by single molecules in solution delivering label-free mass measurements over a broad mass range with high precision and exquisite sensitivity.
Refeyn was founded in 2018 and its instruments are now found in more than 400 laboratories globally. With speed, versatility and ease of use, Refeyn instruments are transforming analytical workflows in laboratories across biopharma – from discovery to manufacture.

Steri-Tek, based in Fremont, CA, with a new facility in Lewisville, TX is a high-volume E-beam/X-Ray contract sterilizer and R&D innovation center serving the medical device, biotech, pharmaceutical and other industries. Steri-Tek is an ISO 11137 and ISO 13485 certified, FDA registered, DEA licensed as well as State of California Medical Device and Drug Manufacturing licensed facility.
The California facility boasts two state-of-the-art 10 MeV, 20 KW linear accelerators. The Texas facility utilizes two state-of-the-art 10 MeV, 30 KW linear accelerators. Both offer simultaneous beam processing that allows for high volume production, providing uniform dose to the product without having to rotate the customer’s boxes. This DualBeam™ configuration significantly increases efficiencies, expands product options, and serves as an effective back-up for the accelerators. Steri-Tek has developed a proprietary system for radiation sensitive materials such as drugs/biologics, combination devices, bioabsorbables, implantables, advanced polymers and other complex products.
Steri-Tek offers;
• Turnkey Validation Services (per ISO 11137 VDmax and Method I)
• Rapid Turnaround on Routine Sterilization Processing (48 hours standard with RUSH 24 hour and 4 hour turnaround available)
• DualBeam™ E-beam/X-ray processing (Highest up time in industry)
• Expert Consultation Services to optimize E-beam/X-ray sterilization processing and throughput of complex devices
Speaker Information
Ben Josey, PhD, Field Application Scientist, Corning Life Sciences
“Interferometric Light Microscopy for Rapid Virus Titering and Characterization of Nanoparticle Preparations: Applications in Downstream Process Development and Sample Normalization”
Abstract:
Significant advancements in upstream biomanufacturing methods for cell and gene therapies have been achieved in recent years. However, final yields continue to be impacted by losses in downstream steps. Interferometric Light Microscopy (ILM) represents a fast and cost-effective method for process development teams to obtain size and concentration data within seconds. This presentation will cover the technical basis of ILM as it compares to other methods for monitoring nanoparticles. Experimental studies developing ILM utilization in characterizing virus preparations, detecting virus breakage and aggregation, and monitoring lipid nanoparticle preparations will be discussed.
Bio:
Ben Josey, Ph.D. is a Field Application Scientist at Corning Life Sciences, where he works extensively with both internal and external research, process development, quality, and manufacturing groups; optimizing cell-based assays, cellular scale-up conditions, instrumentation, and other technical and strategic challenges facing advanced therapies markets.
Peter Holper, BS, Sr. Application Scientist, Biopharma SCIEX
“From Raw Material to Drug Product: Analysis of Therapeutic Gene Therapy Products”
Abstract:
The use of virus and nucleic acid-based platforms for gene therapy delivery in human clinical trials has created the need for robust, highly productive processes for the manufacture of clinical and commercial material. To support this, strong analytics are needed to monitor and characterize these products. Recent developments to improve analytics and process monitoring will be presented and discussed.
Bio:
Peter Holper obtained his degree in Biochemistry and Cell Biology from the University of California – San Diego. Peter has over 15 years of experience in biopharma, including as an analytical chemist at Eli Lilly and Company where he was responsible for developing the analytical control strategy for bioproducts. Peter currently works at SCIEX in Redwood City, CA (US) where he is responsible for developing and optimizing CE applications and providing customer demo sample analysis support.
Maria Oyaski, MS, MA, RAC (US, EU), Regulatory Consultant
“What’s New, and What’s Next, with FDA’s Current Thinking for Cell and Gene Therapies”
Abstract:
The science and manufacturing technologies that enable cell and gene therapies have been advancing rapidly, and FDA has been hard at work generating a level playing field for manufacturers of these complex new products. While some FDA guidance specific to cell and gene therapies has been available since at least 1998, half of the 34 FDA guidance documents in this area have been issued in the last 5 years, and CBER’s new “Office of Therapeutic Products” has announced plans to issue an additional 8 new or revised guidance documents in 2024 (with most of these on manufacturing topics). Recent FDA approvals in cell and gene therapies have also broken new ground on numerous fronts – some of which hasn’t translated into guidance yet.
This talk will present an overview of current FDA thinking specific to cell and gene therapy manufacturing. We’ll focus especially on the 4 guidance documents issued within the past year (Comparability, Potency, CAR-T cell products, Gene Therapy Products incorporating human genome editing), and changes therein versus prior guidance on the same topics (where applicable). The presentation will also discuss apparent trends in the FDA thinking (and especially FDA concerns) related to manufacturing of advanced therapies.
Bio:
Maria Oyaski is a consulting regulatory affairs scientist with 20+ years' experience in development of biotechnology products, including regulatory roles at JDRF, Bavarian Nordic, Heat Biologics, Argos Therapeutics and PPD. She has extensive experience leading teams to productive interactions with FDA to support both allogenic and autologous cell therapies, plus vaccines, other biologics and other medical products. Her regulatory development activities are supported by a decade of experience as a bench scientist in cellular and molecular biology. Maria holds an MS in cellular and molecular biology from the University of Pennsylvania, an MA in History and Philosophy of Science from the University of Pittsburgh, and professional certification in both US and EU regulatory affairs for medical products [RAC (US) and RAC (EU)] from the Regulatory Affairs Professionals Society (RAPS). She is currently president-elect of the North Carolina Regulatory Affairs Forum (NCRAF), and has been active with NCRAF for many years to make professional education easy to access for those interested in learning more about regulatory affairs.
Carmen Amador, BA, Chief Quality Officer, LaSalle Group and Jose Vidal, PhD, CEO, CytoImmune Therapeutics
“Proven Roadmap to Achieve Successful BLA”
Abstract:
The BLA (Biologics License Application) for innovative gene and cell therapies is crucial to go to market, yet a common pitfall for many companies. How do we deliver these therapies to the thousands of patients with life-threatening diseases? Most companies are working around the clock to finish their therapies and get production up and running. Developing a BLA compliance roadmap early on is critical to a successful submission. This effort includes documenting all the processes and procedures in both the development and production of your therapy and challenges the paradigm that GxP is not important at the early stages of development. This session will guide you on how to effectively design your roadmap (including processes, data collection, and documentation) to facilitate and increase the success rate of your BLA submission. By sharing his experience in delivering the first commercially available allogeneic TCell therapy, the CytoImmune Therapeutics CEO, Jose E. Vidal, Ph.D. will share common pitfalls and lessons learned in their journey. Vidal will be joined by former Sr. Director of QA for AstraZeneca, Carmen Amador, who has led over 150 new product and product launches, hundreds of successful BLAs and over 500 regulatory body audits. Together they will discuss the importance of details and developing compliance processes and a quality mindset to establish trust and credibility with regulatory bodies and the healthcare industry. Learn how your team can develop a structured approach to data collection and analysis, documentation, and incorporate scheduled assessments to continuously validate your work and preparedness for BLA submission.
Bios:
Carmen Amador, BS, is a seasoned Quality and Regulatory Affairs executive with over 35 years’ experience in the pharmaceutical, biologics, and bulk-chemical industries, managing multi-national facilities for the manufacture and distribution of prescription, OTC products, biologics, and medical devices. This includes the Quality Operations management of three large manufacturing facilities with a solid record of growth expansions, regulatory compliance, outstanding partnership with FDA & WW regulatory agencies, site validations, and start-up of various new facilities and expansions ranging from 250M to 750M, and the transfer, scale-up, and launch of over 150 new products. Carmen possesses in-depth knowledge of pharmaceutical technologies and their applicable domestic and international regulations, including chemical sterile plants, solids, semi-solids, liquids, injectables, lyophilized products, isolation technology, small molecules, large molecules (biologics), and clinical diagnostic products. Throughout her career, Carmen has demonstrated a proven track record of strategic resource partnering and organizational transformation, leading successful teams in Latin America and European countries. She is driven by a commitment to continuous improvement, developing and optimizing processes, QMS, systems, and workforce development necessary to thrive in a highly competitive landscape. In addition, Carmen is bilingual and proficient in English and Spanish.
Dr. Jose Eduardo Vidal is the CEO of CytoImmune Therapeutics. He obtained his doctoral degree in Cellular and Molecular Biology from the University of Puerto Rico and started his career in the pharmaceutical industry in Wyeth as a process development scientist working with small molecules and the first Biologics development pipeline upon the acquisition of Immunex, where he worked on the development and commercial launch of Mylotarg (very first ADC). He has lead positions of increased responsibility in Tech Services, Process Development and Quality, where he managed 14 sites worldwide. He joined Amgen to lead biologics new product development with over one hundred programs in the pipeline. Jose also led the drug substance manufacturing as the VP of operations and the global late-stage product pipeline and as the global VP of Process Development where he got acquainted with Cell and Gene therapy. In 2018, Jose joined Atara where he led the company as SVP of Process Development and Global Quality to develop and submit for commercial licensing the first ever Allogeneic TCell therapy product (Ebvallo) which was approved for commercial distribution. He joined CytoImmune Therapeutics and has been working in starting up the cutting-edge clinical cell manufacturing from the ground up with operations in PR. CytoImmune is focused on developing oncology and autoimmune malignancies therapies based on Allogeneic NK-CAR cells.
Greg Swan, Business Development, Avantor Sciences
“Demonstration of a critical quality framework for chemical lysis agents through the validation of a novel cell lysis solution that improves vector yield”
Abstract:
Two major challenges for vector-based gene therapies and viral vaccines are the manufacturing of large enough quantities of adeno-associated virus (AAV) needed to drive clinical efficacy and overcoming current process complexity to provide adequate cost-effectiveness. We have dissected the manufacturing workflow and developed impactful and easy-to-adopt unit operations that can be used as a platform for various AAV stereotypes to minimize yield loss and improve overall process yield. One such unit operation is chemical lysis, the industry favored method for the harvesting of AAV for its scalability. However, due to performance and environmental factors, manufacturers are searching for new reagents. This search has emphasized the need for iterative development of tangential processes to find the optimal lysis and particle integrity conditions. We have utilized a critical-quality-analysis framework to demonstrate the benefits of a novel cell solution. Our analysis reveals the benefits of the solution including that it rapidly ruptures cell membranes across a wide range of operating conditions, is compatible with traditional DNA removal agents, and provides preferential high-yield and potency of AAV particles. Through our analysis, we conclude that features of this cell lysis agent make it a prime example of unit operation optimization to improve yield cost in AAV manufacturing.
Bio:
Greg Swan leads cell and gene therapy business development for Avantor. As a trained immunologist with laboratory experience in the cell therapy and viral vector space, he works cross functionally with Avantor’s R&D, product management, and commercial teams to offer consultative support to the CGT space.
Robin Newman, BS, CQV Lead/Project Manager, Core Services Group
“Breakthroughs in Therapeutic Protein Production and Drug Delivery: Defying the Norms of Process Design with Eco-Friendly Reagents and Novel Technologies”
Abstract:
This presentation aims to explore the uses of detergents in viral-vector and LNP therapeutics. It will examine the desired properties of industry-standard detergents, consider long-term outcomes and considerations, and explore process innovations by identifying potential replacements for legacy detergents to achieve safer product and environmental outcomes.
The selection of detergents and solvents is crucial due to their impact on process and product efficiency and quality. Thorough evaluation of detergents and solvents during the product development stages is important because process changes after market entry can affect process validation and initial regulatory filings. When selecting detergents and solvents, several criteria should be considered: (1) the risk of discontinuation influenced by local and foreign environmental regulations, (2) potency, (3) the risk of residuals and the associated removal procedures, and (4) waste product treatment.
Process innovation can help address inherent design risks by developing and studying green alternative detergents or introducing novel process technologies.
The design of the process is guided by heuristics such as process throughput, yield, recovery, and purity. However, factors like capital cost, which determines process feasibility by establishing the facility footprint and selecting and sizing equipment, constrain the forefront of process design. While design principles are often unquestioned, it is important to incorporate social responsibility into these governing factors. The analysis of process inputs and outputs should consider undesirable by-products and outcomes, such as environmental fate and the associated removal processes and waste treatments.
Bio:
Robin Newman is a young and accomplished engineering professional in the Novel Therapeutics Industry. With expertise in start-up ventures, tech transfer, and clinical batch campaign readiness, she brings a wealth of experience to her role. Robin earned a B.S. in Chemical Engineering from Rensselaer Polytechnic Institute. Currently, she serves as a Sr. Process and Validation Engineer at AM Technical Solutions, where she collaborates with a CDMO client to implement cutting-edge AAV platform technology. Beyond her technical prowess, Robin is deeply passionate about patient advocacy, driving her desire to make a significant impact in the field. Her ultimate goal is to become a Novel Therapeutics SME/Principal Engineer, leveraging her expertise to shape the future of the industry.
Andreas Solomos, PhD, Director Virology Operations, CGT, WuXi Advance Therapies
“Cell and Gene Therapy Regulatory Testing; Supporting Products from Development to Market”
Abstract:
Navigating the rapidly evolving regulatory requirements in the field of new cell and gene therapy products is challenging. CTDMO have the distinct advantage of working with a wide array of viral vector products targeting many different of therapeutic indications. Moreover, the T in CTDMO representing testing, plays a crucial role at all stages development from early discovery through market approval and availability. This provides us an opportunity to interface with regulatory bodies around the globe and receive feedback regarding our assays and the data generated for these products. We continually optimize our assays supporting Lentiviral Vectors, Adenoviral Vectors, and AAV Vectors allowing us to support our clients from early development stages with suitable testing programs, setting them up for success throughout their clinical phases and commercial production. To advance the field this knowledge needs to be communicated and feedback regarding safety, potency, and identity testing for viral vectors will be discussed that provide insight into how these assays will support viral vector products throughout the entire development continuum.
Bio:
Andreas manages a team of 60+ scientists and is the director of virology for WuXi's advanced therapies division. Andreas is the SME for audits and regulatory agencies encompassing subjects such as Lentiviral vectors, AAV vectors, Adenoviral vectors, Baculoviral vectors, and Herpes Vectors. Andreas ensures compliance with 9CFR, ICH, FDA, EMEA, PMDA, TGA, and all additional regulations.
Jeff Odum, BS, MS, CPIP, Practice Leader, ATMPS & Biologics, Genesis Engineers
“The Journey to ATMP Commercialization”
Abstract:
The journey to commercialization for many Cell and Gene Therapy products involves many different aspects of the manufacturing enterprise of product-process-facility synergy. Many questions must be answered. Many assumptions will need to be addressed. Changes will occur. This presentation will look at this journey through the lens of companies that are ending, and still navigating this journey, with a focus on providing insight into how to optimize the roadmap.
Bio:
Jeff Odum, CPIP, Practice Lead for ATMPs and Biologics at Genesis, is a globally recognized SME in Biomanufacturing that has over thirty years of experience in the design, construction, and commissioning of facilities in the biotechnology, and pharmaceutical industries. . He is a member of the ISPE Technical Training Faculty and is a Teaching Fellow in North Carolina State University’s BTEC graduate program in biomanufacturing. His ATMP experience includes reference projects in cell and gene therapy, Team Lead for the ISPE Autologous Cell Therapy Good Practice Guide issued in December 2022, Team member for the Allogeneic Cell Therapy Good Practice Guide issued in December 2023, and Developer of the ISPE Professional Training Faculty curriculum on ATMPs.
For questions or more information, contact:
Hannah Cole
Program Manager, Science and Technology Development
Science and Technology Development
919-549-8840
| hannah_cole@ncbiotech.org
UNC Friday Center
100 Friday Center Drive
Chapel Hill, NC 27517

