
CytexOrtho wins first-ever OrthoPitch Technology Competition
CytexOrtho, a Durham-based pre-clinical stage medical device company working to advance orthopaedic treatment options for cartilage repair, has won the inaugural OrthoPitch Technology Competition during the American Academy of Orthopaedic Surgeons (AAOS) Annual Meeting in San Francisco.
Bradley Estes, Ph.D., CEO of CytexOrtho, presented a novel implant designed to naturally restore joints instead of artificially replacing them. The ReNew™ Hip implant offers an innovative solution for the millions of active patients with early hip disease who are too young for a total hip replacement.
The implant technology also can prevent or delay the onset of osteoarthritis (OA), a progressive joint disorder caused by wearing down of cartilage that cushions the ends of bones.

-Photo by AAOS
“This was an incredible event put on by MCRA and AAOS, and I was thrilled to be a part of it,” Estes said. “As we kick the year off with this amazing award and recognition from the Academy, we can’t wait for the next step for CytexOrtho, our first clinical trial, bringing us that much closer to fulfilling the unmet needs of early hip disease patients.”
The AAOS, the world’s largest medical association of musculoskeletal specialists, named CytexOrtho the winner of the inaugural OrthoPitch contest on Feb. 13. The AAOS’s OrthoPitch aims to fuel innovation and foster advancements with the potential to improve or transform existing standards of orthopaedic care.
“The ReNew Hip implant is comprised of two very special components – one is a 3D-woven textile, and the other is a high-precision, Tru3D printed component,” said Estes, during the pitch event. “The integration of these two components give us an implant that not only recreates the form and contour of a healthy articular joint surface but also recreates the function of articular cartilage and bone.”
“Surgeons remove only the damaged tissue and replace it with the CytexOrtho implant,” Estes said. The implant restores the joint to its proper form and contour, and over time, cells move into the gaps in the layers of the implant and form functional tissue while the implant is slowly absorbed into the body. The implant technology received FDA Breakthrough Device designation in early 2023. The company expects to finish its phase 1 clinical trial and launch a phase 2 clinical trial once additional funding is secured.
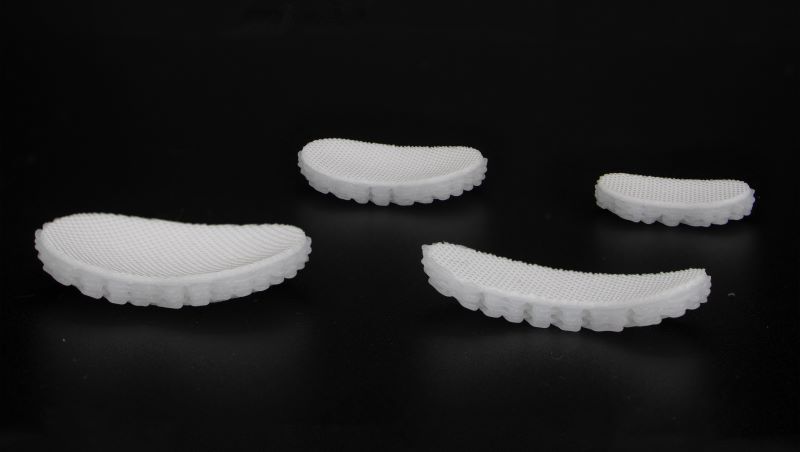
Most of CytexOrtho’s $18.4 million in grant funding to date has come from the National Institutes of Health (NIH). These awards are highly competitive and based on rigorous scientific review. The North Carolina Biotechnology Center (NCBiotech) also awarded the company a Small Business Research Loan of $250,000 in June 2022 to help support activities in preparation for the first-in-human clinical trial for the hip implant.
“I have been working closely with the CytexOrtho team and have found them to be incredibly bright and collaborative,” said Kathy Meserve, senior director of investments, Emerging Company Development at NCBiotech. “Our team at NCBiotech is highly supportive of the company and have been pleased with their technical progress. We were delighted to learn about this well-deserved acknowledgement of excellence by the AAOS.”
Millions of patients in the US who suffer from OA are acutely aware that the condition can often lead to severe joint pain and even disability. Close to half a million hip replacement surgeries are performed in the United States each year, at a total cost of at least $6 billion. And about 92% of them are primarily the result of OA.

“Being named winner of the first OrthoPitch competition validates the dedication and hard work our team at CytexOrtho has devoted to helping patients through this technology,” Estes said. "We've brought together top-tier orthopedic research scientists, industry veterans in orthopedic devices, and leading hip preservation surgeons to bring this product to market. It's now time to deliver the solutions these patients have long been anticipating."
Presented by AAOS and sponsored by MCRA, OrthoPitch kicked off in July 2023 with a call for companies to submit a new technology or product concept with a focus on its impact on the practice of medicine and patient care. The AAOS Devices, Biologics and Technology (DBT) Committee conducted a multi-staged review process focused on ease to market, reimbursement coverage, impact on market and commercialization.
The top four submissions, including CytexOrtho, were invited to pitch their concept live to a distinguished panel of regulatory, reimbursement and commercialization experts, physicians, and investors during the AAOS Annual Meeting. Following the pitches, the audience witnessed a spirited round of questions from the panel of judges. The judges and the audience, which contributed to the outcome via a live online vote, named CytexOrtho the winner.
“Orthopaedic surgeons are at the forefront of new procedures and technologies to optimize patient care in an evidence-based approach,” said Jason L. Dragoo, MD, FAAOS, and DBT Committee chair. “With programs such as OrthoPitch, we're able to nurture innovation(s) from the ground up and witness energy and excitement for the future of musculoskeletal care. The experience of being in person at the AAOS Annual Meeting gave new meaning to 'pitching a deal' and allowed each finalist the opportunity to receive valuable feedback.”
As grand prize winner, CytexOrtho will receive:
- Exposure to top seed and early-stage investors physicians, strategists, and fellow industry members
- A complimentary booth at AAOS 2025 Annual Meeting in San Diego
- Complimentary regulatory or reimbursement assessment by a MCRA consultant
- One seat access to Axiom Health's Market Intelligence Platform
- One 20-minute Innovation Theater time slot at the AAOS 2024 and 2025 Annual Meetings
- A complimentary webinar with AAOS
- One-on-one coordinated meetings with a minimum of nine leading MedTech investors
