
FDA OKs Novartis Flu Vaccine
 |
|
| Novartis vaccine production at Holly Springs facility. (Courtesy of Novartis Vaccines and Diagnostics) |
North Carolina’s reputation as “Vaccine Central” got an official boost from federal regulators Tuesday with marketing approval for Novartis’ flu vaccine using the highly efficient process of cell culture.
The United States Food and Drug Administration put its blessing on the innovative product named Flucelvax, making it the first influenza vaccine of its kind available in the U.S.
The most unique feature of the product is the fact that it’s grown in dog cells rather than fertilized chicken eggs. In the event of a flu pandemic, at least 25 percent of the nation’s vaccine protection can be quickly produced at the $1 billion, 167-acre, six-building Novartis Holly Springs campus.
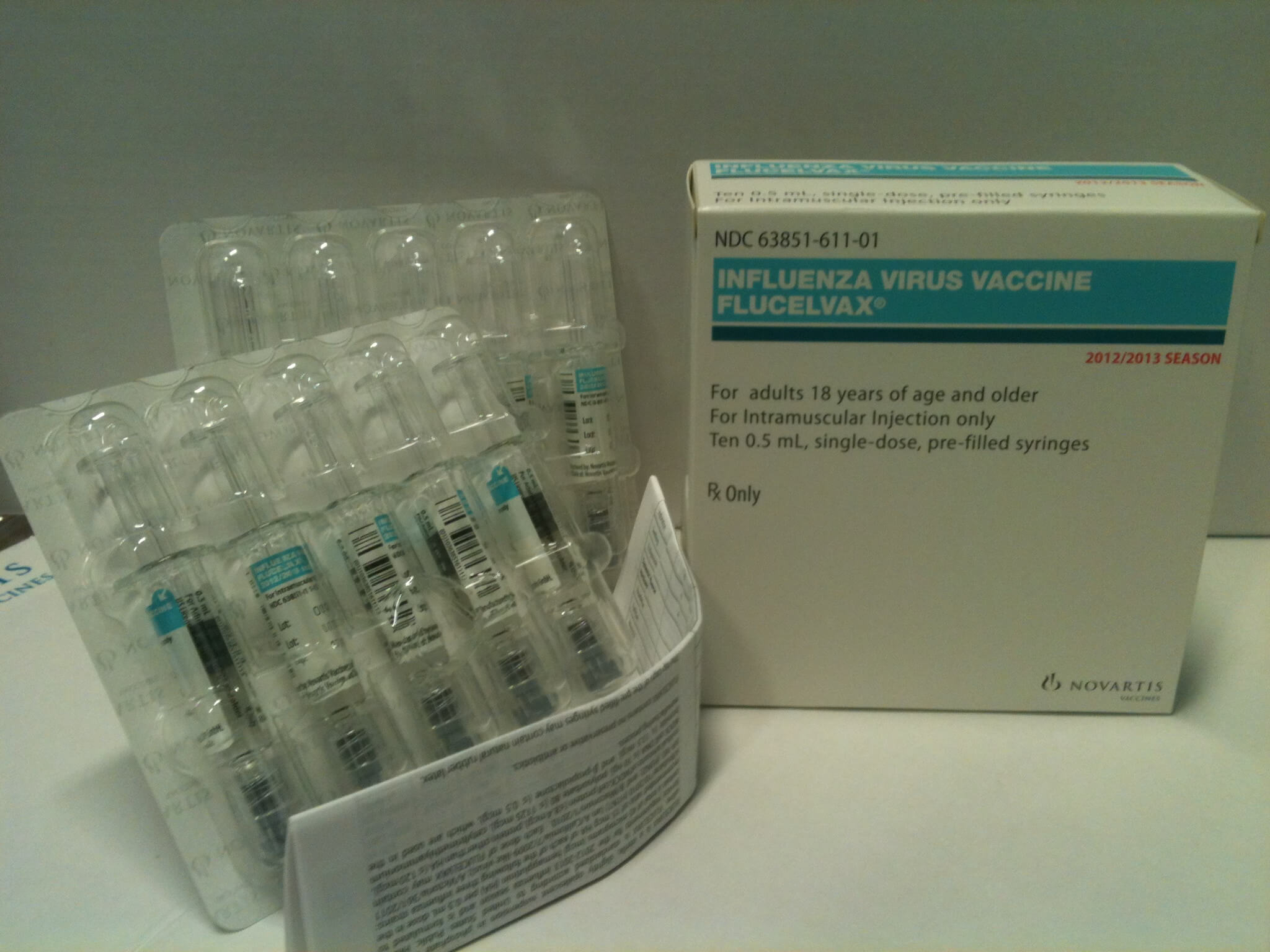 |
| Flucelvax, Novartis' newly approved flu vaccine for the U.S. market |
The U.S. Department of Health and Human Services’ Biomedical Advanced Research and Development Authority gave Novartis a $289 million contract to support the design, construction, validation and licensing of the plant.
The resulting facility, 25 miles south of Research Triangle Park, is the largest biomanufacturing plant in the U.S. It now employs some 500 people, more than 85 percent of whom were drawn from North Carolina’s well-trained workforce. The workers occupy more than 430,000 square feet of manufacturing, laboratory and office space.
NCBiotech helps recuitment effort
Novartis, headquartered in Basel, Switzerland, decided in 2006 to build the plant in North Carolina after absorbing California-based Chiron. Recruitment specialists from the North Carolina Biotechnology Center and partner organizations worked with Chiron, and then Novartis, successfully demonstrating the state’s readiness to accommodate the factory.
The first employees started working at the facility in 2009, and it has consistently met quality and production milestones ever since.
 |
BTEC provides major workforce training
Novartis has worked closely with North Carolina State University’s Biomanufacturing Training and Education Center (BTEC), the statewide BioNetwork community college program and other specialty training programs in North Carolina to deliver the company’s highly skilled workforce.
BTEC is training scientists from other countries to use cell culture-based manufacturing techniques similar to that used in the Holly Springs facility. The training program is part of a World Health Organization initiative to strengthen the ability of developing countries to produce flu vaccine, potentially reducing the global threat from influenza.
The Holly Springs facility also produces a vaccine “booster” ingredient, called an adjuvant, that helps excite the body’s immune system to provide more protection from invading pathogens with less vaccine. Novartis uses an adjuvant derived from shark’s livers, though company officials emphasize that sharks are not killed for that purpose.
Another unique flu vaccine production facility, established in RTP by the Canadian firm Medicago, is growing immunity-producing viral-like particles in the cells of tobacco leaves rather than in eggs or mammalian cells. That $42 million factory has received $21 million in federal funding, and appears poised to provide the fastest turnaround of vaccines, at lower cost, of all current production technologies.
