
Scinovia Developing Blood-flow Imaging System for Heart Bypass Surgeries
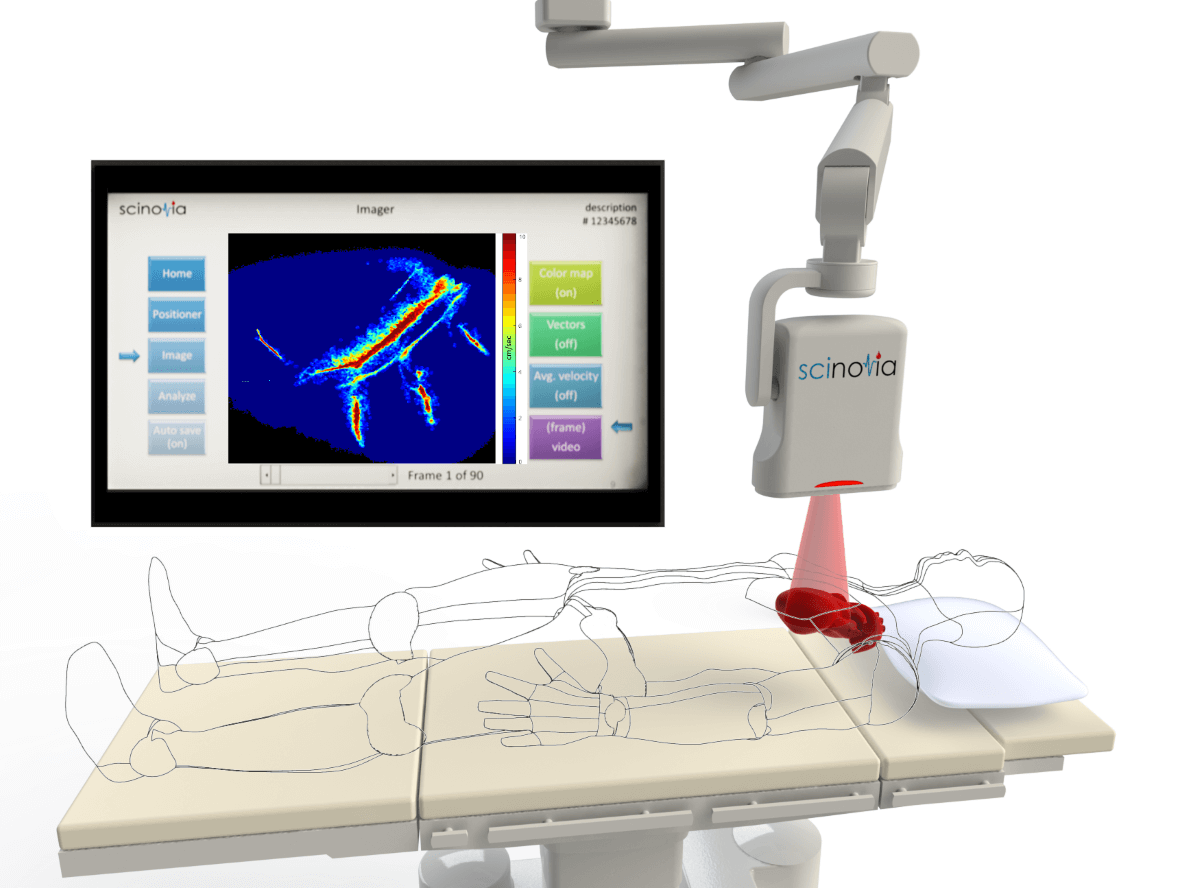 |
| Rendering of ceiling-mounted version of Scinovia's real-time non-invasive blood flow display system for use in heart surgeries. -- Scinovia photos |
By Barry Teater, NCBiotech Writer
Durham-based Scinovia Corp.’s new imaging system to help surgeons monitor patients’ blood flow during operations is moving closer to the market in 2016.
Scinovia, founded in 2014, has three employees and a dozen contractors. Its technology was developed by a team of medical technology professionals assembled by Jim Sund, Ph.D., Scinovia’s founder and CEO, from the alumni ranks of the three major Triangle-area universities: Duke, North Carolina State University and the University of North Carolina at Chapel Hill.
In 2015 the company raised $600,000 from private investors in a Series A financing round, and it plans to raise $6 million more in a Series B round to drive commercialization, says Sund.
Scinovia will test a mobile prototype in animal surgeries at the NCSU School of Veterinary Medicine in March and then have 10 surgeons on its medical advisory board be the first to test it in clinics and hospitals throughout the United States.
The system is a non-invasive, mobile device that enables surgeons to visualize blood-flow speeds in real time, verify their work, and make corrections if necessary, Sund says.
Initially targeting heart bypass surgeries
More than three million surgical procedures each year could benefit from the technology, he says, but Scinovia will focus initially on the largest market adopters, the 700,000-plus heart bypass surgeries performed annually around the world.
A recent study in the Journal of Cardiothoracic Surgery revealed that 20 percent of graft bypasses have insufficient blood flow. One-third of global bypass surgeries now use a quality assurance (QA) tool to verify adequate blood flow in new graft vessels, and the adoption rate for these QA tools is growing by 11 percent per year, Sund says. The market is about $100 million and is projected to reach $200 million by 2021.
One commonly used QA tool relies on ultrasound probes to measure the speed of blood flow at a single point. Another uses an injected dye to visualize where blood can flow over the point of care. These QA tools require precious time and physical contact with delicate vessels or injection of a chemical dye.
 |
| Jim Sund, Ph.D. |
“Scinovia’s device combines the value of both QA tools without patient contact by using trade-secret algorithms, lasers, and an advanced imaging system to deliver more clinically actionable information,” Sund says.
Buyers of Scinovia’s system will be hospitals and clinics that provide heart bypasses, plastics, transplants, removal of necrotic tissue, and other surgeries where risk of blood flow problems may delay patient recovery, he says. The system can be ceiling mounted in an operating room or be used on a mobile stand.
The system will be tested in various model systems and in live animal studies, but should not require human clinical trials before commercial launch, Sund says. This is because so-called “predicate” devices already exist and are viewed as information-enhancement tools that supplement surgeons’ sensory abilities.
“We simply need to show up in the market to capture a substantial base of adopters,” Sund says.
The company plans to file a regulatory submission with the Food and Drug Administration a year into Series B funding that would clear the system for sale as a Class II device.
Maps will help healers know the flow
After the system is in wide use, Scinovia plans to collect quantitative blood-flow maps from before and after bypass surgery. The maps will be useful in developing predictive analytics to help the medical community improve health outcomes, Sund says.
“Hospitals can show positive results to insurance payers to help avert Medicare’s new penalties for excessive re-admissions,” he says.
Sund has a cross-disciplinary background in business strategy and execution, commercialization, life science, physical science and engineering. He holds a Ph.D. in engineering and an MBA from Duke and earned a bachelor’s degree in chemistry and zoology from the University of Florida.
