
NC Researchers, Companies Help Lead Coronavirus Battle
The rapid global march of the coronavirus that began in Wuhan, China several months ago could be slowed by researchers and biotech firms 7,770 miles away – in the Research Triangle of North Carolina.
The urgency and significance of this scientific endeavor has suddenly hit home, as a Raleigh resident this week became the first North Carolinian to be diagnosed with the highly publicized COVID-19 infection.
Scientists from the state’s academic community – along with companies from its biotech sector – are playing an important role in understanding how the coronavirus works and developing new drugs to attack it.
The University of North Carolina at Chapel Hill’s Gillings School of Global Public Health is a case in point. Researchers there have studied coronaviruses for several decades now. They want to better understand and more effectively treat these types of diseases that range from the common cold to COVID-19, the new one associated with the novel SARS-COV-2 virus currently infecting populations around the world.
Coronavirus outbreaks aren’t new, of course. Severe acute respiratory syndrome –or SARS – surfaced in 2002. It resulted in 774 deaths in 17 countries, a mortality rate of about 10%.
A decade later, Middle East respiratory syndrome (MERS) emerged. It infected about 2,000 people. More than 700 of them – about 36% – died.
By comparison, COVID-19 – while more contagious – currently appears to be fatal in only about 2% to 3% of cases. But that’s still much higher than influenza, which has a mortality rate of about 0.1%. The Centers for Disease Control and Prevention estimates that between 12,000 and 61,000 people in the United States have died from the flu each year since 2010.
Ralph Baric, Ph.D., Kenan Distinguished Professor in the Department of Epidemiology at the Gillings School, says the recent COVID-19 epidemic “looks like it’s transitioning into a full-blown global pandemic.”
The Baric, Heise and NCBiotech collaborations
Baric is one of the country’s leading experts on coronaviruses. He operates the Baric Lab at the Gillings School, which specializes in these diseases and other emerging infections. His research uses various models to determine how viruses move among species and cause disease.

The COVID-19 epidemic isn’t Baric’s first rodeo. He’s studied coronaviruses for more than three decades, including SARS in early 2002 and MERS in 2012. During that time, he’s been involved in both drug and vaccine development.
Baric has worked closely over the years with another UNC scientist, Mark Heise, Ph.D. – an influenza expert. They’ve collaborated on several large research projects to better understand how viruses cause disease, and then to develop improved vaccines and anti-viral strategies to help prevent them.
Heise is a professor of genetics at the UNC School of Medicine. The Heise lab is focused on understanding how diseases result from interactions between viruses and the hosts they infect.
Heise said he and Baric began working together during the original SARS outbreak in 2002 and 2003. They’ve used the primary viruses they study – influenza and coronavirus – as comparators. “We come at the research from two different directions and meet in the middle,” he added.
The North Carolina Biotechnology Center has awarded several grants to support both scientists over the years. The most recent came this year in the form of a $190,340 Pfizer Gene Therapy Fellowship to support the research of Victor Tse, Ph.D., in Baric’s lab. Tse is exploring whether the benign adeno-associated virus, used by scientists to deliver gene therapies into the body, can be used to deliver antivirals to protect against coronavirus infections.
NCBiotech is also soliciting grant proposals from research scientists throughout the state working on some aspect of the battle against the novel SARS-COV-2 virus. These Flash Grants provide up to $20,000 to energize the most creative ideas that exhibit early indications of commercial potential.
COVID-19 antiviral treatment has roots in NC
Two of Baric’s colleagues at the Gillings School also have played important roles in recent coronavirus research. Timothy Sheahan, Ph.D., and Amy Sims, Ph.D., led a successful study a couple of years ago to test the potential of a small-molecule inhibitor to fight outbreaks of the illness.

Sheahan is a research assistant professor in the Department of Epidemiology and Sims was a research associate professor there. Sims has subsequently taken a faculty position at the Pacific Northwest National Laboratories in Washington. Sheahan also has received support from NCBiotech.
Their study was part of a grant run out of the University of Alabama in partnership with UNC, the Vanderbilt University School of Medicine and pharmaceutical company Gilead Sciences, Inc. Researchers from the Gillings School generated much of the data. And the findings led Gilead to develop remdesivir, an antiviral medicine currently under evaluation in coronavirus patients in China and the U.S. It’s considered by some to be the leading candidate to treat COVID-19.
The therapy – originally identified as GS-5734 – proved effective against several coronaviruses in mouse studies, as well as the Ebola virus. “We tested this drug against multiple genetically distinct coronaviruses and it worked great against them all,” Sheahan said when initial data were released in 2017. “The results make us optimistic that this drug...could be used for any new coronavirus that emerges in the future.”

A handful of COVID-19 patients in the United States recently received remdesivir on a compassionate use basis, which allows doctors to try experimental drugs when no other options are available. A clinical trial is now underway at the University of Nebraska Medical Center.
The first patient to receive remdesivir was a passenger who returned to the U.S. after testing positive for coronavirus aboard the Diamond Princess cruise ship. Others diagnosed with COVID-19 who have been hospitalized also will be part of the study.
Remdesivir clinical trials are taking place in China as well. Results may be available as early as April. Since the drug has already proven safe, it could theoretically be available for patient use in a matter of months, if it’s shown to be effective.
“This is a real illustration of how important our research here at UNC and in North Carolina is in responding to this most recent coronavirus outbreak,” Heise said.
Other NC scientists, biotech companies also in the race

At Duke University, Gregory C. Gray M.D., MPH, FIDSA is involved in overseeing a clinical trial of a vaccine to combat the SARS-CoV2 virus.
Gray is an infectious disease epidemiologist and professor with three affiliations: The Division of Infectious Diseases in Duke University’s School of Medicine, the Duke Global Health Institute, and the Duke Nicholas School of the Environment. He also serves as a professor in the Program in Emerging Infectious Diseases and the Global Health Institute at Duke-NUS Medical School, Singapore and as a professor of global health at Duke Kunshan University in China.
Another Duke physician scientist, Matthew Rubach, like the other academic researchers in this story, has received funding related to coronavirus work from the National Institutes of Health in recent years.
Rubach, a specialist in clinical infectious diseases with medical training in pediatrics, internal medicine and medical microbiology, is co-director of the Kilimanjaro Christian Medical Centre-Duke University Health Research Collaboration in Moshi, Tanzania.
At least a half dozen North Carolina companies also are working on drugs and diagnostics to detect and combat COVID-19.
Burlington-based LabCorp has launched a diagnostic for the COVID-19 infection, allowing physicians to order and administer the test, with analyses provided by LabCorp within three to four days.
Heat Biologics filed a new provisional patent application March 3. The Morrisville company wants to apply its immune system activating technology for the treatment and prevention of infection to SARS-COV-2 – the virus that causes COVID-19.

The company has focused mainly on treating cancer, but recently shifted toward coronavirus. NCBiotech recruited Heat Biologics to the state in 2011 and awarded the business a $225,000 Strategic Growth Loan.
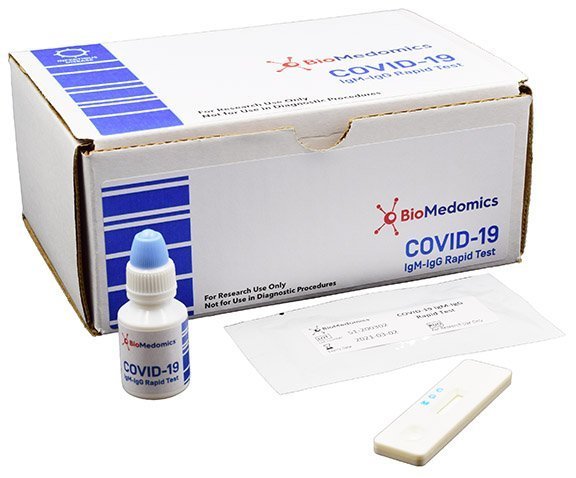
A Durham diagnostic test kit producer that was bootstrapped with two loans from the Biotech Center, BioMedomics, has developed a COVID-19 test kit that is being used by researchers in various countries and by health officials in China. Using a small blood sample in a test strip, it can diagnose the infection in about 15 minutes. The company is hoping to work with U.S. health officials to gain the approvals needed for marketing the kit in this and other countries.
A new Cary-based startup, Atom Bioworks, plans to use artificial intelligence and synthetic DNA to create specific pattern-matching nanostructures from which to identify and treat coronaviruses.
Company co-founder Sherwood Yao said Atom Bioworks’ Pattern-matching Enabled Sensing and Therapeutics – or PEST – technology can provide more accurate, versatile and rapid COVID-19 testing, at a much lower cost. It also might be used to disrupt the disease itself so that patients’ immune systems can fight the virus.
The technology currently is in the research stage, according to Yao.
Durham-based BioCryst is developing a broad-spectrum antiviral therapy shown to be active against more than 20 RNA viruses, including coronaviruses. That’s according to John Bluth, senior vice president of investor relations and corporate communications.
Bluth said the experimental drug, galidesivir, will be made available to U.S. public health authorities as they consider different approaches for treating and preventing COVID-19.

And CSL, the Australian parent company of Holly Springs-based Seqirus, said it has partnered with the University of Queensland on that institution’s coronavirus vaccine initiative. CSL will offer technical assistance and provide Seqirus’ proprietary adjuvant technology – MF59 – to the preclinical development program.
Adjuvants are used in vaccines to create a stronger immune response and to speed vaccine development and output.
Seqirus is the world’s largest producer of cell-based influenza vaccines.
Moderna Therapeutics, a Cambridge, Mass., biotech with no North Carolina roots, is set to become the first company to begin a human trial of an investigational vaccine for COVID-19. It has shipped the initial batches of its mRNA-1273 to the National Institutes of Health for a phase 1 clinical trial, to begin in April.
Vaccines will likely face a much longer road to approval and availability, though. It could take a year to 18 months or more to get a new vaccine on the market.
Currently there are no approved COVID-19 therapies. But the good news is that “compared to the SARS outbreak 16 or 17 years ago, the progress and speed with which the whole field is moving to find new treatments is pretty impressive,” Heise pointed out. “This is a testament to the fact that we’ve continued to work on understanding these viruses since the original SARS outbreak. And it’s also a reflection of how much more capable the scientific community is than we were in the early 2000s.”
