
TransEnterix Receives FDA Clearance For Senhance Ultrasonic System

TransEnterix, a medical device company located in Research Triangle Park, has received clearance from the U.S. Food and Drug Administration to market its Senhance Ultrasonic System.
Senhance is the first abdominal robotic surgery platform since 2000 to receive FDA approval. And it’s the only digital laparoscopic system to use haptic force feedback (the sense of touch generated by applying vibrations or motions to the user), which allows physicians to feel the force surgical instruments generate when handling delicate tissue. Senhance also offers reusable instruments that help keep costs in line with more traditional laparoscopic procedures. In addition, it uses 3-millimeter instruments for microlaparoscopic procedures that result in nearly scarless incisions in patients.
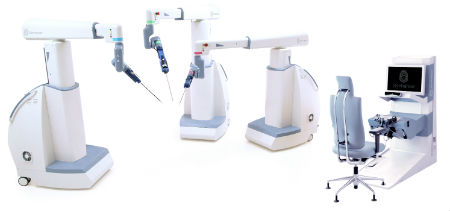
-- TransEnterix photo
“The addition of ultrasonic technology is a significant expansion of the Senhance system capability preferred by many surgeons during complex procedures,” said Steven McCarus, M.D., FACOG, chief of gynecologic surgery at Florida Hospital Celebration Health. “Combining advanced energy tools with the precision control, haptics and ergonomics of the Senhance digital interface may allow many surgeons to confidently use this technology across the broadest range of pathology and patients.”
TransEnterix said Senhance technologies set the direction for future robotic surgery by improving the patient-surgeon experience, while also lowering costs. “We believe the addition of the Senhance Ultrasonic System is significant and broadens the attractiveness of the platform and digital laparoscopy for surgeons in the U.S.,” said TransEnterix President and CEO Todd Pope. “These instruments deliver controlled energy to effectively ligate and divide tissue and minimize thermal injury to surrounding structures.”
Senhance is approved in the U.S. for laparoscopic colorectal, gynecological, inguinal hernia and cholecsystectomy (gallbladder removal) surgery. It’s also available in Europe and other countries.
TransEnterix uses digital technology to improve minimally invasive surgery by tackling the clinical and economic challenges associated with current laparoscopic and robotic options.
