
Polarean Trial Exceeds Expectations, Company Seeking Pre-NDA Meeting

A successful Phase 3 trial of Polarean Imaging’s drug-device combination for lung imaging has the Research Triangle Park company poised to seek next-step advice from FDA regulators.
Polarean, a spinout of Duke University, is pursuing clinical use of a three-dimensional technology that has been limited to research labs so far. But the company says its clinical importance is in its ability to improve images of patients’ lung function and capacity using a whiff of a harmless gas called hyperpolarized 129Xenon during magnetic resonance imaging (MRI). The Polarean method then uses three-dimensional mapping to monitor respiratory function.
In conjunction with MRI, the inhaled hyperpolarized gas assesses lung function and capacity with Xenon scintigraphy. This technique provides a unique and sensitive way to monitor changes in lung structure and function.
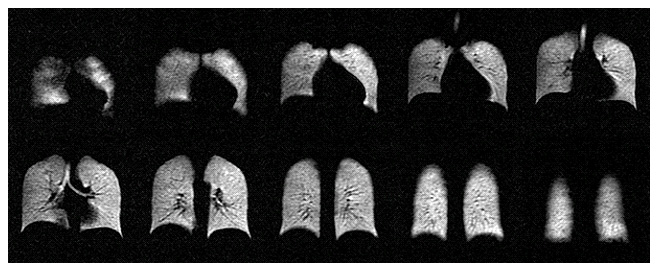
“Given the limitations of existing methods to diagnose and monitor lung disease, we see a significant unmet need for non-invasive, quantitative and cost-effective image-based diagnosis technology without exposing patients to ionizing radiation,” said Richard Hullihen, CEO of Polarean. “We believe that our technology has the potential to overcome these limitations and we look forward to using data from the clinical trials to support our New Drug Application.”
After positive results from multi-center human testing on patients receiving surgical removal of damaged lung tissue and on patients receiving lung transplants, Polarean is seeking a pre-NDA meeting with FDA officials to discuss the drug-device combination before submitting a New Drug Application.
Polarean designs and manufactures equipment for production of hyperpolarized xenon or helium gas. When used in conjunction with MRI, these gases offer a fundamentally new and non-invasive functional imaging platform. Current investigational uses include identifying early diagnoses of respiratory diseases as well as monitoring progression and therapeutic response. In addition, xenon gas exhibits solubility and signal properties that enable it to be imaged within other tissues and organs.
The company launched in January 2012, after securing all of GE Healthcare’s assets in the field of hyperpolarized gas MRI, including the exclusive rights to approximately 30 patent families. Polarean systems are installed at academic research institutions worldwide, including the United States, Canada, UK, Germany, and Sweden.
They’re currently used in basic and clinical research to study lung physiology and to monitor the efficacy of new drugs.
