
NC's Gilero Uses Ingenuity, 3D Printing to Expand COVID-19 Protective Equipment, Ventilators

Using 3D printers, Morrisville medical device manufacturer Gilero has begun a three-tiered response to the shortage of COVID-19 protective equipment for healthcare workers.
“As a company with medical device expertise, we’re really trying to see what we can do to help,” said Kaitlyn Shaffer, Gilero’s marketing and communications manager, in an interview.
The 50-person company has about one-third of its staff still working in its Morrisville labs and its Pittsboro manufacturing plant and the other two-thirds working from home. Founded in 2002, Gilero designs, develops and manufactures products for the medical device and drug delivery device markets, and deals with associated regulatory issues.
Shaffer said the company staff continues working on its regular projects and volunteers additional time weekends and nights to the COVID-19 efforts.
In the first tier of its COVID-19 battle plan, it is developing immediate designs for improvised face shields and simple facemasks. It will provide open source CAD and instructions for making a 3D printed face shield using readily available materials such as a two-liter soda bottle. It has also designed an improvised facemask using fabric from a cotton t-shirt.
Gilero will 3D print a number of face shield components and assemble them in its Pittsboro manufacturing facility.

Shaffer said the company realizes the cotton masks do not meet Centers for Disease Control and Prevention (CDC) standards for health care worker protective equipment. Anecdotal evidence suggests they may offer some protection if medical-grade masks are not available.
In the event of personal protective equipment shortages, “Something is better than nothing,” Shaffer said.
Tier two of Gilero’s response is to develop and manufacture reusable respirators and goggles in partnership with its operation in China. It is using its mold building and injection molding capabilities for each of these designs. The respirator will consist of an injection molded mask with filtration equivalent to N95 requirements that can be easily replaced.
The mask itself will be fitted to the face and can be disinfected using standard disinfectants. By developing a reusable alternative to the disposable N95 mask, it hopes to help combat shortages while offering healthcare professionals the same or better levels of protection as a single-use respirator. The project is expected to take a few weeks, Shaffer said.
The third tier of Gilero’s approach is to develop an emergency ventilator that can be powered by pressurized air sources found at most hospital beds or portable air compressors, as well as a standard wall power outlet. This is a collaborative effort involving Gilero engineers skilled in medical device design, Adam Steege, founder of Trio Labs, and Huade Tan, a life support systems engineer.
If it can reasonably demonstrate that this design is safe, effective, and meets all of the requirements by the various solicitations that have been issued, the company said it will make the design available for download and construction by anyone skilled to do so, in an open-source format.
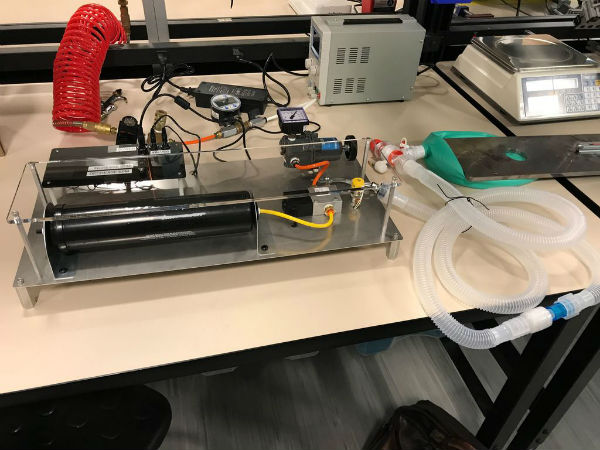
Shaffer noted that Gilero is also offering additional ventilator manufacturing capacity in its newly renovated medical device manufacturing facility in Pittsboro. “We have available manufacturing space (both cleanroom and white room), and the necessary quality systems and FDA registration. Should a ventilator supplier with capacity limitations need assistance, we are making our facility and employees available to help.”
Gilero will be accepting contributions of filter media for respirators, TPE materials, and a number of electronic and mechanical components. Access to additional 3D printers would also be valuable, Shaffer said. While Gilero is funding the first two tiers of this plan, it will seek outside funding for the emergency ventilator.
“We’re in the process of setting up a non-profit organization for donations and a few generous entrepreneurs have already indicated they are willing to donate to the ventilator project,” Shaffer said.
Gilero does not plan to patent, or profit from, any of these designs. Instead, it aims to make these designs and assembly instructions, backed by its medical device expertise, freely available to anyone.
