
NCBiotech-boosted Satsuma Aims to Head Off Migraines

Migraine sufferers know every second counts when trying to head off an arduous event by taking medication.
The last thing they need to do is assemble a device if they have impaired thinking or vision, or depend on oral medications while nauseated or vomiting, all common symptoms at onset of an attack.
Satsuma Pharmaceuticals, Inc. (Nasdaq: STSA), a clinical-stage biopharmaceutical company headquartered in South San Francisco, with operations in both California and Research Triangle Park, completed a $90.8 million initial public offering in September. It will use the money to develop its acute treatment for migraine that addresses all these issues.
STS101 (dihydroergotamine (DHE) nasal powder) is a fast-acting, patient-friendly formulation of DHE, which has been effectively used for migraine since 1946 by injection in a clinic or hospital setting. Satsuma’s formulation allows fast, easy access to the drug from pocket or purse.
It also makes DHE available outside a clinical setting to patients who don’t do well taking pills.
Triptans are a class of tryptamine-based drugs for use to abort an acute migraine attack, which do not work optimally for many people with migraine, according to John Kollins, Satsuma’s president and CEO. DHE has been shown to have advantages over triptan therapies and to work in patients who have failed triptans.
The STS101 difference
Satsuma’s Phase 1 clinical trial demonstrated STS101’s rapid absorption with clinically relevant drug concentrations within 10 minutes and sustained levels over time comparable to those achieved by intramuscular DHE injection. Importantly, no subjects administered STS101 experienced nausea or vomiting, which are common side effects of DHE when administered by intravenous injection.
“We are excited to report that the intranasal administration of STS101 in our Phase 1 clinical trial achieved a PK (pharmacokinetics) profile that is favorable in comparison with other non-injectable DHE formulations, including liquid nasal spray and pulmonary-route orally inhaled products, said Detlef Albrecht, M.D., chief medical officer at Satsuma.
Migranal, marketed by Bausch Health Companies, Inc., is a liquid nasal spray formulation of DHE. However, according to Kollins’ research and the drug’s prescribing information, absorption of Migranal is highly variable. Data on the Migranal website indicates time to symptom relief is significantly longer (almost half of trial participants still had no relief after 2 hours). Also, it is more cumbersome to carry and requires assembly of a sprayer to vial and priming of the sprayer before administration. This is while a sufferer is experiencing debilitating symptoms at onset of a migraine attack, including pain and/or visual disturbances, light sensitivity and difficulty concentrating.
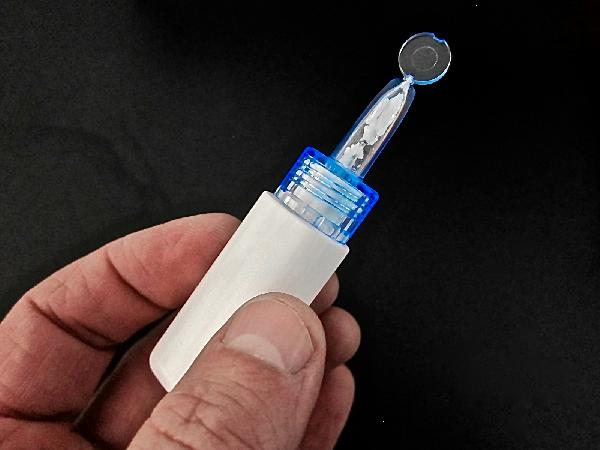
Of note, the active ingredient, DHE, in both Migranal and STS101, as well as the triptans, is not indicated for the prevention of migraine, only treatment at onset of an acute attack.
“With the dry powder, we’ve shown you can get more drug in faster,” said Kollins. “You can administer the full dose of STS101 in a matter of seconds. It is dramatically simpler to use.”
In addition, Kollins said – and Migranal’s prescribing information confirmed – its liquid form of DHE is sensitive to light and heat. It must be stored at 77 degrees Fahrenheit or less. This impedes the portability of carrying it in a purse, pocket or backpack on a warm day.
“The powder form of DHE is much more stable,” Kollins said.
Satsuma’s Phase 3 Emerge Study to evaluate the efficacy and safety of STS101 is the largest-ever trial with a DHE product, said Kollins. Enrollment began in July 2019 with estimated primary completion date in the second half of 2020. He said the company expects to file a New Drug Application with the U.S. Food and Drug Administration at the end of 2021.
More than a bad headache
Migraine is a neurological disease involving nerve pathways and brain chemicals with extremely incapacitating neurological symptoms during “attacks” that usually last between four and 72 hours. The sooner a sufferer can abort the process of an acute attack with medication, the shorter its duration and, often, the less severe the symptoms.
During an attack, sufferers may experience severe, throbbing recurring pain in one or both sides of the head, visual disturbances (flashing lights or squiggles of blurry vision called aura), nausea, vomiting, dizziness, extreme sensitivity to sound, light, touch and smell, and tingling or numbness in the extremities or face, according to the Migraine Research Foundation. More than 90% of sufferers are unable to work, attend school or function normally during a migraine attack.

Nearly one in four U.S. households includes someone with migraine, including women, men and children. The condition tends to run in families. Women disproportionately suffer from migraine and represent 85% of chronic sufferers. Chronic migraine means headache occurring on 15 or more days per month for more than 3 months.
Migraine accounts for an estimated $36 billion annually in healthcare and lost productivity costs. This includes more than 157 million workdays lost each year in the U.S. and untold “presenteeism,” during which someone remains at work with low or no productivity. Children with migraine miss school twice as often as children without migraine.
A large range of lifestyle, environmental, weather-related, hormonal, medication, food or allergic triggers can cause onset of a migraine attack and vary according to individuals. Women can experience migraines with fluctuations in hormones during monthly cycles, pregnancy, nursing, while taking oral contraceptives and menopause.
Research continues to increase understanding of what causes migraine. Older theories suggested symptoms resulted from fluctuations in blood flow to the brain. Current researchers regard changes in blood flow and blood vessels not as the initiators of pain, but contributors. Instead, there is wide understanding that chemical compounds and hormones, such as serotonin and estrogen, play a role in pain and the constriction or dilation (expansion) of blood vessels, which, in turn, contribute to pain and other symptoms, according to Johns Hopkins Medicine.
About Satsuma
Satsuma Pharmaceuticals is a spinout company from the Japanese firm Shin Nippon Biomedical Laboratories (SNBL), headquartered in Tokyo. SNBL is a global preclinical and clinical contract research services provider and creator of innovative therapeutic technologies. SNBL created the proprietary dry-powder nasal formulation and drug delivery device technologies incorporated in STS101.
Satsuma closed a $12 million Series A funding round co-led by RA Capital Management and TPG Biotech in January 2017. It closed a $62 million Series B round of funding in April 2019 with participation by existing investors (RA Capital Management, TPG Biotech, and SNBL) and new investors, Osage University Partners, CAM Capital, Surveyor, Eventide Asset Management, Cormorant, Lumira Ventures and SBI Investment.
In late September, after closing its IPO, Satsuma moved to offices in Research Triangle Park from the spaces it had been renting at the North Carolina Biotechnology Center’s Landing Pad. The Landing Pad program offers prime physical office space and a full cadre of professional services to cushion companies’ landing as they establish a footprint in North Carolina’s life science hub.
Kollins said Satsuma has eight employees at its new RTP location, which is more than the number at its San Francisco location, including Robert Schultz, vice president and head of chemistry, manufacturing and controls; Mic Iwashima, vice president and head of operations; and Shannon Strom, Ph.D., R.A.C., vice president and head of regulatory affairs. Schultz and Strom came from Pearl Therapeutics in Durham (acquired by AstraZeneca), where they worked on metered-dose inhalation products for respiratory indications. Iwashima, a North Carolina State University engineering graduate, has 15 years’ experience with SNBL developing the nasal drug delivery platform and product candidates.
The company anticipates hiring several more North Carolina employees and currently is seeking a device engineer.
“We have access to two talent pools,” said Kollins, referring to the benefits of the company’s East Coast-West Coast U.S. presence. Furthermore, he said he also has personal and professional ties to the Triangle region. His undergraduate degree in engineering is from Duke University. He also wanted to place the company near others working on inhalation delivery products to be able to tap into that expertise.
