
Mycovia Drug Shows Promise Against Drug-Resistant Yeast Infection

Durham-based Mycovia Pharmaceuticals, a developer of drugs in women’s health, has shown that its oral drug candidate VT-1598 is active against Candida auris, an emerging multi-drug resistant fungal pathogen that presents a serious global health threat.
The U.S. Centers for Disease Control and Prevention (CDC) recently deemed Candida auris a global emerging threat. This recently discovered yeast strain typically strikes both men and women in hospitals with compromised immune systems, and contributes to the growing health threat associated with drug-resistant infections.
NovaQuest Capital Management formed Mycovia in 2018 following NovaQuest’s acquisition of Viamet Pharmaceuticals. It recently acquired VT-1598, which Mycovia is developing to combat serious fungal pathogens. The results of the study on Candida auris were published in the March issue of Antimicrobial Agents and Chemotherapy, a journal of the American Society for Microbiology.
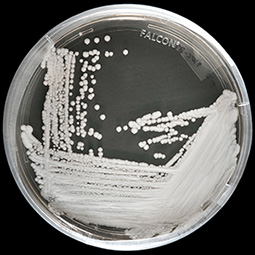
-- CDC photo
Susceptibility testing was conducted by the Fungal Reference Laboratory at the CDC where VT-1598 demonstrated test tube (in vitro) activity against 100 clinical isolates of Candida auris.
Treatment with VT-1598 also resulted in significant and dose-dependent improvements in survival and reduction in kidney and brain fungal burden compared to control in mice infected with Candida auris. The company said these results suggest that VT-1598 may be a future option for treating invasive Candida auris infections.
“As a women’s health organization, Mycovia is passionate about developing breakthrough drugs in areas of significant unmet medical needs,” said Patrick Jordan, CEO of Mycovia and partner at NovaQuest, in announcing the findings. “Concurrently, we realize that there is tremendous potential for oral fungal inhibitors to treat multi-drug resistant fungal pathogens such as Candida auris which is virulently spreading across the globe,”
The company’s lead product candidate, oteseconazole, is a novel, oral therapy for recurrent vulvovaginal candidiasis (RVVC). RVVC is commonly known as a chronic yeast infection that affects millions of women each year, and is often accompanied by severe physical discomfort and emotional burden.
“While women’s health remains our number-one priority as we move through Phase 3 clinical trials with the goal to be the first FDA-approved treatment for recurrent vulvovaginal candidiasis,” said Jordan, “we continue to assess and commence new opportunities where Mycovia can address tremendous unmet medical needs.”
