
Lucerno Launches Massive PET/CT Scan Tracer Study
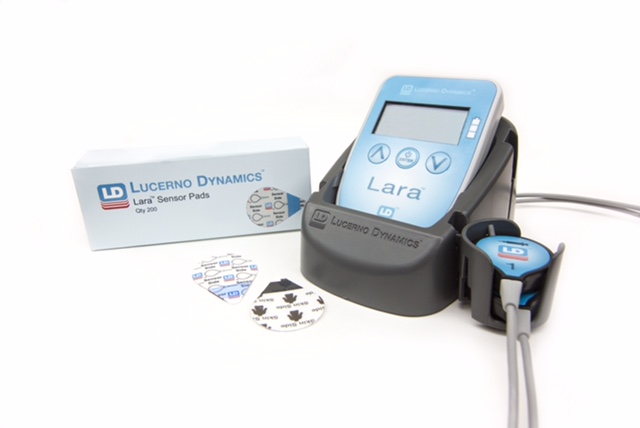 |
| Lucerno's Lara system for monitoring effectiveness of radiotracer injections for patients undergoing diagnostic PET and CT scans. -- Lucerno photo |
Lucerno Dynamics, a Morrisville company developing noninvasive sensor technology that can show the effectiveness of radiotracer injections in patients undergoing PET/CT scans, has launched a nationwide study to demonstrate the benefits of its system.
The study, called “Lara-QI,” is to enroll more than 10,000 patients in up to a dozen sites around the country during 2017. A $300,000 Strategic Growth Loan from the North Carolina Biotechnology Center provided funding for the study. Lucerno said it’s the world’s largest quality improvement study ever conducted on the radiotracer injection process.
The company, founded in 2011, was awarded a $47,000 NC IDEA grant in 2012. In early 2014 the Biotech Center awarded Lucerno a $150,000 Small Business Research Loan to help it develop and test its technology.
A year later, NCBiotech awarded the company a $3,000 Industrial Internship grant to support the work of East Carolina University dentistry student Ryan Le, MBA, in developing a business plan for its system.
In 2016 the company raised $6.34 million in Series A funding.
To produce the highest quality PET/CT images, a prescribed dose of radiotracer is injected all at once into a patient’s vein and circulation for about an hour of “uptake” before a patient is imaged, said Ron Lattanze, CEO of Lucerno. “Any issue with the quality of the injection may compromise the image. Often times, the physician reviewing the image may be completely unaware that the injection was flawed.”
Nationwide study has two key goals
The Lara – QI study, which will employ Lucerno’s recently FDA-listed Lara System, aims first to assess the quality of participating centers’ radiotracer injection processes and then will assess centers’ ability to improve quality. Lara uses patient-friendly pads with small lightweight sensors on a patient’s arms during the injection process, and adds less than one minute to the patient’s imaging experience.
David Townsend, Ph.D., co-inventor of the PET/CT scanner and Lara – QI co-principal investigator, said it is important to monitor the quality of a PET/CT scan, particularly when the outcome of the scan has implications for assessing response to cancer therapy and subsequent management of the patient.
“Lucerno’s Lara sensors can assess the quality of the injection process, removing at least one potential source of variability from the scan protocol with important implications for the patient,” said Townsend, who serves as director of the Singapore Clinical Imaging Research Centre and professor of radiology at the National University of Singapore.
Lara – QI co-principal investigator Terence Wong, M.D., Ph.D., professor of radiology and chief of the Division of Nuclear Medicine at the University of North Carolina School of Medicine, explained that patients’ anatomy and differing techniques from one PET/CT center to another can cause variability in the injection process.
“Today there is no reliable way for nuclear medicine physicians or radiologists to assess the quality of the injection process as they review a patient’s PET/CT image,” Wong said. “Initiating and completing the Lara – QI study is an important step toward understanding what happens during a patient’s uptake period and could help us improve the quantitative capabilities of PET/CT imaging.”
