
Locus Biosciences Makes This Year’s Prestigious ‘Fierce 15’

The Fierce 15 may sound like a movie title, but it’s an annual listing of the biotechnology industry’s most promising private companies. That’s according to FierceBiotech – the world’s largest and most read biotechnology news publication.
The 2019 FierceBiotech assemblage includes a familiar name from the Research Triangle area. Morrisville-based Locus Biosciences, a developer of precision antibacterial therapies, joins 14 other companies on the list from the U.S., Belgium, the U.K. and Spain.
“This year has seen unrivalled scientific talent in the early stage life sciences world,” said Ben Adams, senior editor at FierceBiotech. “Each of these companies brought something different, exciting and potentially life-changing for myriad patients around the world across a host of diseases and disorders.”
Locus is no exception. It’s developing what is known as Type 1 CRISPR-Cas3-enhanced bacteriophage therapeutics. Locus starts with naturally occurring bacteriophages (phages) that infect and replicate within bacteria. It then increases their ability to kill bacteria by arming them with CRISPR-Cas3. The technology doesn’t touch the non-targeted “good’ organisms in the patient’s body.
CRISPR phage technology is death to bad bacteria cells
CRISPR – the acronym for Clustered Regularly Interspaced Short Palindromic Repeats – is a naturally occurring immune system that bacteria use to fight off infection. In recent years, scientists have adapted one form of CRISPR – Cas9 – to edit DNA at specific places in the genome.
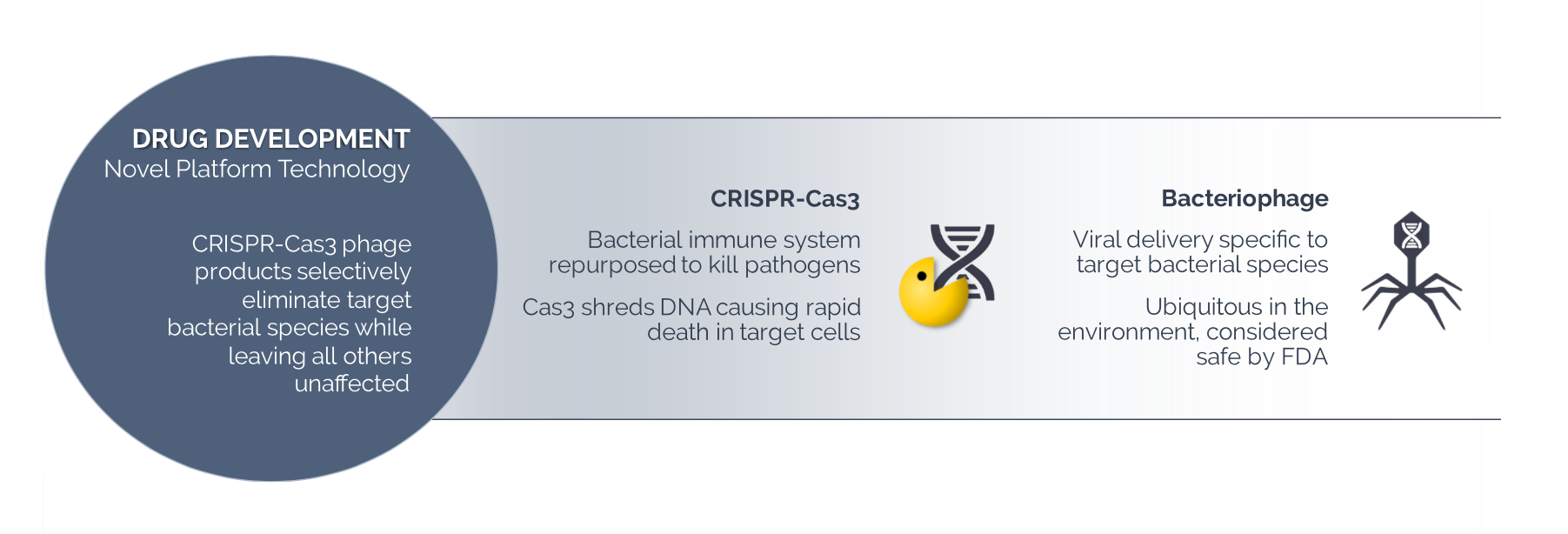
Locus’ introduction of CRISPR-Cas3 is important because, unlike Cas9, it shreds the target DNA, leading to cell death. That helps destroy residual bacteria that could otherwise survive infection by the bacteriophage.
Locus CEO Paul Garofolo explains it this way. Phages can target and attack bacteria, but “just like any other predator-prey relationship on the entire planet, a predator will never kill all its prey.” Natural phages always leave residual bacteria behind and that can provide a pathway for a pathogen to come back even stronger. CRISPER-Cas3 finishes the job.
The idea of using phages to fight bacteria isn’t new. They’ve been deployed for that purpose since the late 19th century, but usually weren’t effective enough on their own to treat serious infections. So the approach was relegated to the back burner with the successful introduction of antibiotics.
Now antibiotic resistance and the emergence of superbugs – along with the booming field of research that connects particular bacteria with diseases such as inflammatory bowel disease, autism and Alzheimer’s – have caused scientists to take a fresh look. A number of phage companies have emerged, but Locus is unique in its use of CRISPR-Cas3 to increase the effectiveness of phage therapy. It hopes to expand and improve the treatment options for antibiotic-resistant bacterial infections and other large-market microbiome-related diseases.
The beauty of the Locus technology is twofold. It’s a powerful tool to fight infections. Yet it’s expected to be safe for healthy cells because a phage only binds to specific bacteria. “Even if the drug product circulates through the human body...it will only interact with the targeted set of bacteria, and all the other bacteria in the body are safe,” Garofolo said. That is dramatically different than the way antibiotics work.”
Locus Biosciences off to a promising start
It’s a promising start for a company that opened its doors only four years ago as a North Carolina State University spinout. Locus was founded with the help of a $75,000 Company Inception Loan from the North Carolina Biotechnology Center. The Center followed up with a $250,000 Small Business Research Loan. And three of the four scientific founders of Locus also have received NC Biotech grants totaling more than $300,000.
At the beginning of this year, the company inked a major deal with Janssen Pharmaceuticals, a division of Johnson & Johnson. The agreement is worth up to $818 million. It gives Janssen the exclusive license to develop, manufacture and commercialize CRISPR-Cas3-enhanced products to potentially treat two pathogens that infect the respiratory tract and other organs.
