
Humacyte Starts Phase 2 Trial of Bioengineered Blood Vessel
By Barry Teater, NCBiotech Writer
 |
|
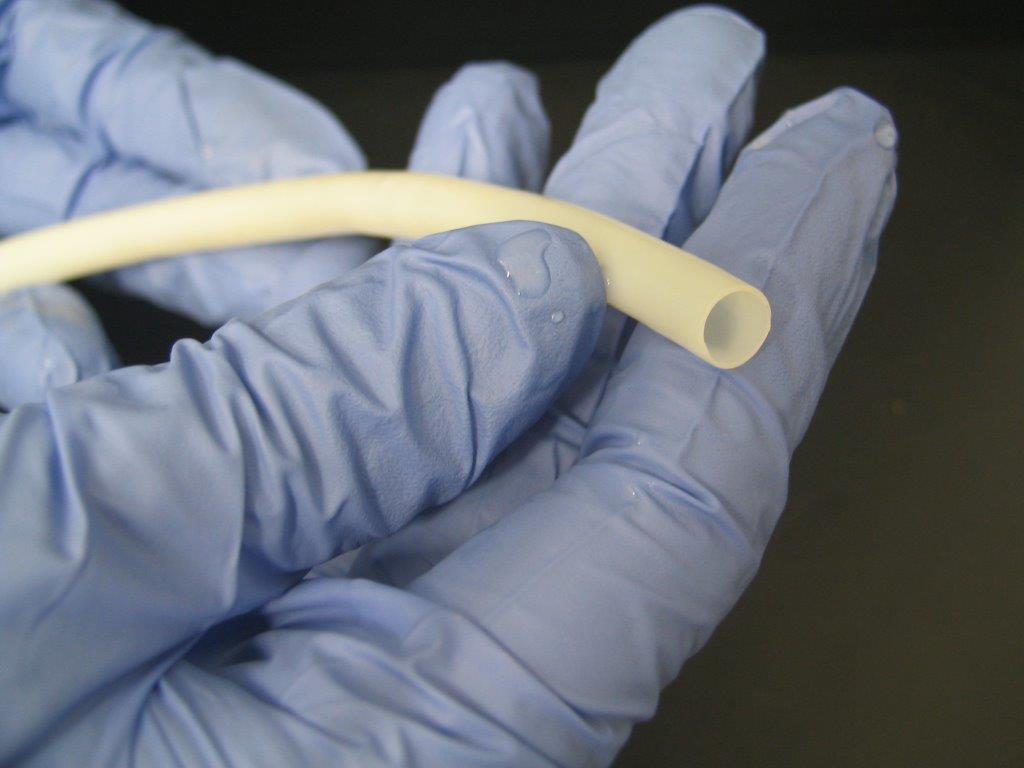
Humacyte's Humacyl vessel made from regenerative medicine technology. -- Humacyte photo |
Humacyte, a regenerative medicine company based in Morrisville, has launched a Phase 2 clinical trial of Humacyl, its bioengineered blood vessel, in patients with peripheral arterial disease.
The non-randomized trial will test the safety and efficacy of Humacyl as a lower extremity arterial bypass vessel in about 20 patients over the next 12 months, the company said in a news release. Humacyl will be surgically implanted above the knee in an attempt to improve blood circulation.
The study goal is to assess whether the vessel performs in the arterial bypass position and is usable and suitable for repairing arterial blood vessels.
The U.S. trial will follow previous arterial trial surgeries that were completed at multiple sites in Poland in 2015. Humacyl is also undergoing a Phase 3 clinical trial for vascular access in patients with end-stage renal disease who require renal replacement therapy but are not candidates for fistula, a surgery that joins veins and arteries.
“We are heartened by the fact that Humacyte is expanding its footprint by leveraging our first-in-class, bioengineered vessel for multiple vascular surgery applications ̶ including patients that undergo hemodialysis and patients with peripheral vascular disease,” said Jeffrey Lawson, M.D., Ph.D., chief medical officer of Humacyte. “The continuation of Humacyl’s Phase 2 clinical studies as a conduit for blood flow in a patient with peripheral arterial disease marks a major milestone in the field of regenerative medicine.”
Humacyte enrolled its first U.S. patient in the trial for arterial bypass at Brigham and Women’s Hospital in Boston under an FDA-approved protocol. Patients will be enrolled in at least three other sites in the United States, including Duke University in Durham, the UCSF Medical Center in San Francisco and the Michigan Vascular Center in Flint, Mich.
“Over 8.5 million people in the United States suffer from peripheral arterial disease,” said Michael Belkin, M.D., chief of the division of vascular and endovascular surgery at Brigham and Women's Hospital. “If the results of this clinical trial are positive, then this solution has the potential to serve as a new clinical option for the many patients that face the need for artery bypass surgery each year. We are pleased to be the first medical facility in the United States that is taking part in a key study that may lead to a meaningful impact on patients’ cardiovascular health.”
Peripheral artery disease is a narrowing of the arteries to the legs, stomach, arms or head, restricting or blocking blood flow. The disease is caused by atherosclerosis, a buildup of fat and cholesterol deposits, or plaque, in the artery wall.
Humacyte was spun out of Duke University in 2004 by scientists Laura Niklason, Shannon Dahl and Juliana Blum.
The privately held company received a $150,000 Small Business Research Loan from the North Carolina Biotechnology Center in 2006. In October 2015 it raised $150 million in a Series B preferred stock financing ̶ among the largest ever by a life science company in North Carolina.
In August 2016 Humacyte received a $9.9 million investment from the California Institute for Regenerative Medicine (CIRM) to support the Phase III clinical trial of Humacyl for kidney disease.
Humacyl is derived from the company’s proprietary cell-culture technology for engineering human, extracellular matrix-based tissues that can be shaped into tubes, sheets or particulate conformations, with properties similar to native tissues. These investigational tissues, called human acellular vessels, have many potential uses in regenerative medicine and vascular surgery as off-the-shelf products, the company said.
Humacyl received Fast Track status from the U.S. Food and Drug Administration in 2014, accelerating the regulatory review process.
