
FDA to Review Liquidia's Inhaled Lung Hypertension Therapy

The U.S. Food and Drug Administration has accepted Liquidia Technologies’ new drug application for its pulmonary arterial hypertension treatment, LIQ861.
The FDA expects to complete its review by December.
Liquidia, of Morrisville, creates a wide range of therapies using its proprietary PRINT technology. The technology is a particle engineering platform that enables production of highly uniform drug particles with precise control over their size, shape and chemical composition.
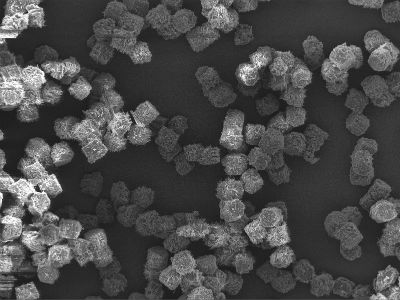
LIQ861 is an investigational, inhaled dry powder formulation of treprostinil, a synthetic analog of prostacyclin. It’s a vasoactive mediator essential to normal lung function that is deficient in patients with PAH.
Liquidia says LIQ861’s PRINT formulation safely delivers higher doses of the vasodilator into the lungs.
"FDA acceptance of this NDA is a significant milestone for our company and our PRINT technology,” said Neal Fowler, chief executive officer. “PRINT was the cornerstone that allowed for the precise engineering and development of LIQ861 into its current form and serves as the foundation for all of our products in development.”
Pulmonary arterial hypertension is a chronic, progressive disease caused by the hardening and narrowing of the pulmonary arteries that can lead to right heart failure and eventually death. It is estimated that approximately 30,000 patients across the U.S. suffer from the disease. The exact cause of PAH is often unknown. Though the symptoms are treatable, there is no known cure for the disease.
