
Duke Gets Grant to Develop ‘Fast, Simple, Low-Cost’ COVID-19 Test
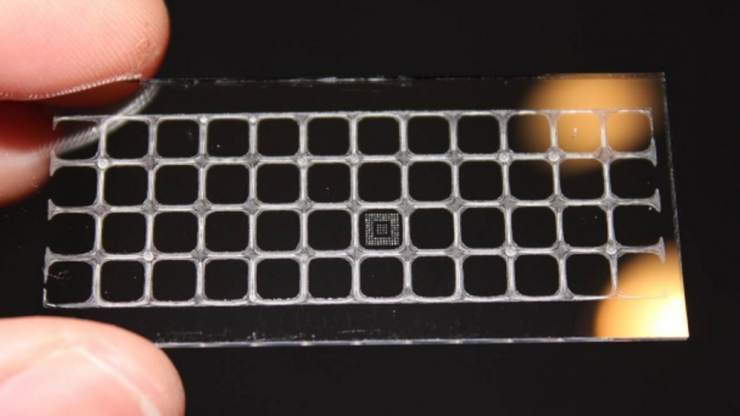
Duke University biomedical engineers are hoping a rapid testing platform originally designed to detect Ebola can now be used to catch antigens to COVID-19.
Now thanks to a $119,000 grant from the National Science Foundation, the team is getting to put it to the test.
If it works, it could potentially shorten the wait time for coronavirus testing from days or hours down to just 30 minutes, said principal investigator Ashutosh Chilkoti, the Alan L. Kaganov Distinguished Professor of Biomedical Engineering at Duke.
The diagnostic tool, called the D4 assay, is a self-contained, inkjet-printed test on a small glass slide. It can detect low levels of antigens, the protein markers of a disease, from a single drop of blood or from a throat or nose-swab sample.
“We’ve shown a proof-of-concept by detecting a biomarker of the SARS-CoV-2 virus (which causes COVID-19), and the next step would be to validate this with patient samples,” Chilkoti said. “Our test is designed to be truly point-of-care, and this pandemic is clearly a scenario when a portable, fast and cost-effective diagnostic would be most useful.”
Two types of antibodies
The D4 assay tool has two types of antibodies printed on its surface: detection antibodies tagged with a fluorescent marker; and capture antibodies, which are primed to find the antigens specific to a pathogen.
When a sample is placed on the slide, the detection antibodies separate from the array and bind to the target antigen proteins in the sample. These antibody-antigen complexes then attach to the capture antibodies on the slide, which fluoresce in response to the connection.
A handheld scanner is then used to look for lights indicating the presence of the antigen.
Unlike other antigen diagnostic tests, Chilkoti said, the D4 assay is printed on a polymer brush coating, which prevents non-target proteins from attaching to the slide’s surface. This removes any “background noise” on the chip, making it easier to detect low levels of the target proteins.
Chilkoti said they hope to start testing the technology with COVID-19 patient samples in the next few months. It would be in collaboration with Chris Woods, a clinical investigator and professor of global health and medicine and Chief of the Infectious Diseases Division at Durham’s VA Medical Center, across the street from Duke.
However, human testing depends on FDA approval.
“Challenging times also create opportunities,” Chilkoti said. “Given that this is a huge societal and public health challenge, those of us who create new technologies have a responsibility to act, and we’re grateful that the support from the National Science Foundation allows us address this challenge head-on.”
