
CivaTech’s Radiation Device Tested in First Pancreatic Cancer Patient
By Barry Teater, NCBiotech Writer
 |
A Virginia man has become the first person in the world to be treated for pancreatic cancer with a bio-absorbable, implantable radiation device developed by Durham-based CivaTech Oncology.
The device, called CivaSheet, was surgically implanted in 70-year-old William Grubbs Jr. of Varina, Va., in March to treat his early stage pancreatic cancer.
The doctors who performed the surgery at Virginia Commonwealth University’s Massey Cancer Center in Richmond, Va., reported no complications six weeks after the surgery, according to accounts of the procedure published by VCU and public radio station WCVE.
Since that successful surgery, another patient at VCU has also been treated successfully with CivaSheet, said Suzanne Troxler Babcock, the company’s executive chairman and CEO.
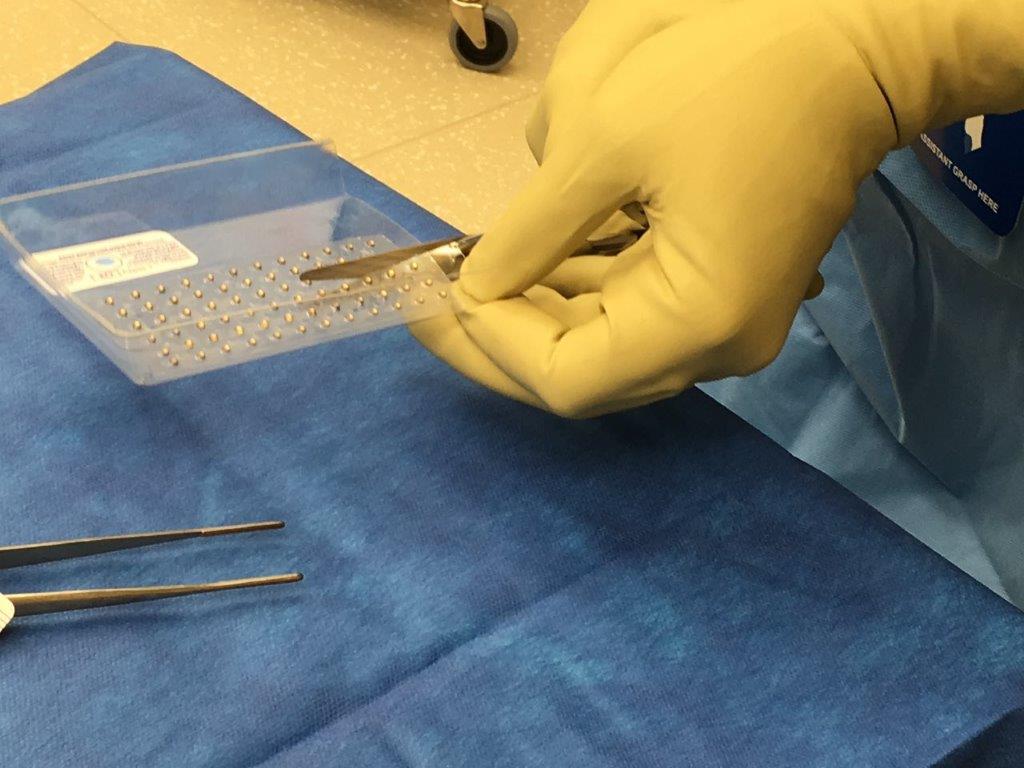
CivaSheet is a thin, flexible, polymer membrane that contains a radioactive isotope, Palladium-103, on one side and a gold foil barrier on the other side. When surgically implanted, the active side of the CivaSheet emits radiation therapy toward cancerous tissue while the other side blocks radiation from harming healthy tissue.
The product was developed with support from the North Carolina Biotechnology Center, which provided CivaTech with a $249,048 for a Small Business Research Loan in 2012.
 The company has registered the trademark “Gold is Cold” to remind clinical staff that the gold side of CivaSheet is the shielded side of the product, Babcock said.
The company has registered the trademark “Gold is Cold” to remind clinical staff that the gold side of CivaSheet is the shielded side of the product, Babcock said.
The goal of the low-level radiation therapy is to help minimize cancer cell recurrence in the pancreas.
CivaSheet is implanted in a 15-minute procedure at the end of a longer surgery to remove the diseased portion of the pancreas. After it has delivered its radiation over about three weeks, the device is mostly absorbed by the body within 50 to 70 days, and no follow-up procedure is needed to remove it, according to the company.
CivaSheet is a form of internal radiation therapy, also known as brachytherapy, in which radiation is concentrated at a short distance to kill cancerous cells. Brachytherapy is used to treat several cancers throughout the body but has not been used for pancreatic cancer until now.
In 2016 an estimated 53,000 people were diagnosed with pancreatic cancer in the United States, according to the American Cancer Society. Of those, about 20 percent were candidates for the potentially lifesaving “Whipple” surgery in which the diseased portion of the pancreas is removed.
Limits collateral tissue damage
Standard of care includes radiation treatment, but radiation oncologists are limited in the dose they can give to patients due to the proximity of healthy tissue and critical organs to the affected area and the toxic side effects resulting from unintended radiation dose to these areas from other radiation treatment methods. The CivaSheet bypasses this limitation by allowing the surgeon to place the CivaSheet directly on the pancreas where it can deliver as much radiation as required without the collateral tissue damage.
The ability to customize the directionality of radiation using CivaSheet will help radiation oncologists treat a variety of other cancers such as soft tissue sarcoma, early stage non-small-cell lung cancer, head and neck cancer, colorectal cancer, ocular melanoma, and skin cancer, CivaTech said in a 2014 press release announcing FDA clearance of CivaSheet.
New clinical studies on tap
CivaSheet is available to patients at VCU as an experimental option. A Fast Track NIH-sponsored clinical trial to assess vascular toxicity and a Phase 2 trial to determine the rate of cancer growth after one year are expected to begin soon.
In this multi-center study, 80 patients undergoing the Whipple surgical procedure will be given a highly targeted dose of radiation by an implant of the CivaSheet, directly to surgical margins that are prone to cancer recurrence. The study will track the effectiveness of this new approach in preventing recurrence.
CivaSheet is designed for use either during surgery, as was done at VCU recently, or with standard, less invasive, implant devices.
CEO cites NCBiotech's 'important role'
“The North Carolina Biotechnology Center plays an important role in nurturing small technology companies,” Babcock said. “Their staff has been very helpful in a number of business areas, and they have the pulse of the biotech community locally and across the state. We have trusted their recommendations and appreciated the programs and referrals."
CivaTech has also received research grants from the National Cancer Institute.
The company has another brachytherapy product called CivaString. Like CivaSheet, it contains Palladium-103 embedded in a polymer but in the form of strings that are injected by needle. CivaString is intended to deliver a more uniform dose of radiation than traditional metal seeds.
CivaString was granted clearance by the FDA for therapeutic use in localized tumor sites and has been used to treat prostate cancer since 2013.
CivaTech has 16 employees and expects to add more at its home office and manufacturing site in Durham this year, Babcock said. The company also has an office in Atlanta.
Prior to co-founding CivaTech, Babcock was a vice president at Troxler Electronic Laboratories in Research Triangle Park, where she led strategic corporate growth and innovation including R&D, product development and product launches.
