
bluebird bio Launches Gene Therapy for Severe Blood Disorder in Germany

bluebird bio, a gene therapy company with operations in Durham, has launched for the first time its treatment for a severe inherited blood disorder, in Germany.
LentiGlobin, marketed as ZYNTEGLO, is a one-time gene therapy that addresses the underlying genetic cause of transfusion-dependent β-thalassemia (TDT), an inherited blood disorder caused by a mutation in the β-globin gene. It interferes with red blood cell production, leading to severe anemia.
Until now, TDT patients faced a lifelong regimen of blood transfusions to survive and suppress symptoms of the disease. These transfusions often led to serious complications and organ damage from iron overload.
This new therapy, however, will potentially eliminate or reduce the need for blood transfusions.
“This milestone enables the first commercial treatment of ZYNTEGLO for TDT patients in the European Union (EU),” Alison Finger, bluebird’s chief commercial officer, told the North Carolina Biotechnology Center.
A completely new treatment
bluebird is working with its manufacturing partner, apceth Biopharma GmbH, to commercially manufacture ZYNTEGLO in the EU.
It has also collaborated with the University Hospital of Heidelberg to become the first qualified treatment center to administer the therapy.
In addition, bluebird has entered into value-based payment agreements with multiple statutory health insurances in Germany to help ensure patients and their healthcare providers have access to ZYNTEGLO.
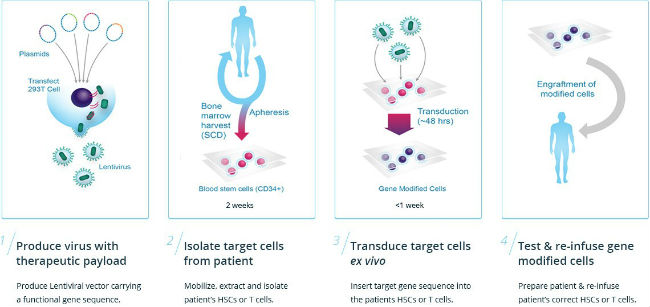
“Gene therapy is a completely new treatment paradigm, and, as a result, regardless of country, evolution of health systems are required in order to support patient access to one-time treatments,” Finger explained.
“Conversations with other governments and payers in the EU are ongoing. bluebird continues to work closely with all stakeholders to ensure patient access and affordability for gene therapies.”
The European Medicines Agency previously granted Priority Medicines (PRIME) eligibility and Orphan Medicinal Product designation for ZYNTEGLO to treat TDT. ZYNTEGLO is also part of the agency’s Adaptive Pathways pilot program, intended to improve timely access for patients to new medicines
ZYNTEGLO has also been granted Orphan Drug status and Breakthrough Therapy designation for treating TDT by the U.S. Food and Drug Administration. However, it is not yet approved in the U.S.
bluebird bio has initiated the rolling biologics license application (BLA) submission for approval in the U.S., and is engaged with the FDA in discussions regarding the requirements and timing of the various components of the rolling BLA submission.
“Subject to these ongoing discussions, the company is currently planning to complete the BLA submission in the first half of 2020,” the company said in its release.
Durham facility opened in 2019

bluebird is a publicly held company headquartered in Cambridge, Mass., with facilities in Durham, Seattle, and Zug, Switzerland.
The Durham facility is a 125,000-square-foot manufacturing plant that bluebird purchased in 2017 and opened last year.
The site produces lentiviral vector for the company’s investigational gene and cell therapies, including: bb2121 and bb21217 to treat multiple myeloma, and potentially LentiGlobin to treat TDT and sickle cell disease.
bluebird employs approximately 82 scientists, engineers, manufacturing and operations personnel at the facility.
