
Humacyte Gets $10M Boost for Clinical Trial
By Barry Teater, NCBiotech Writer
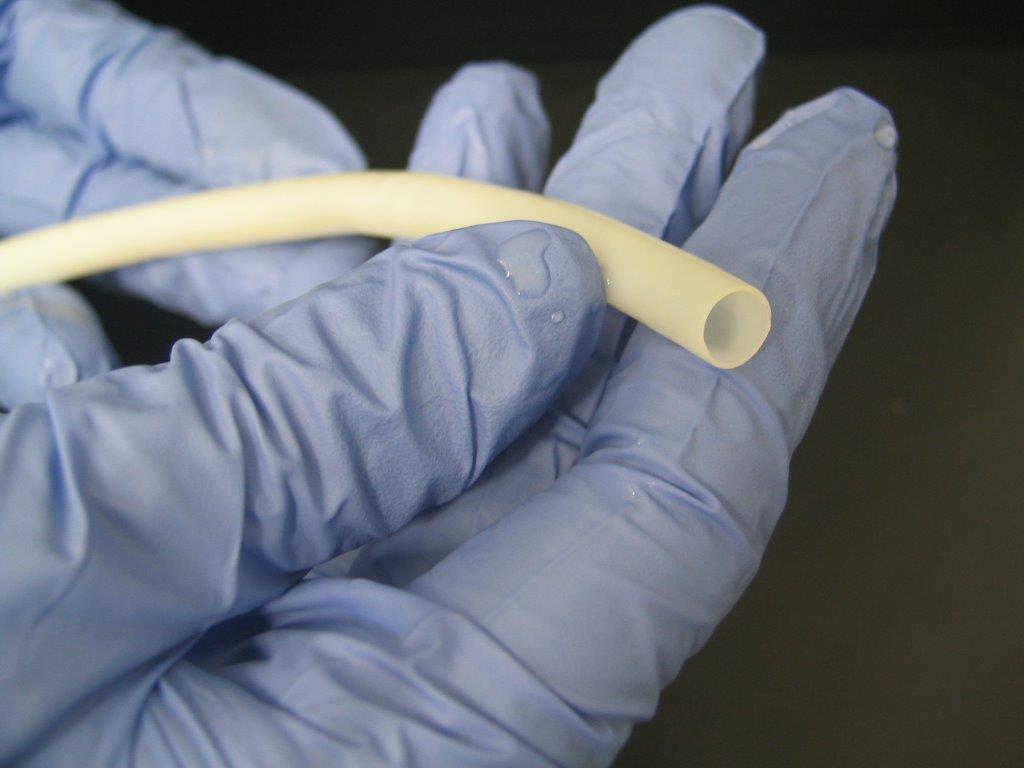 |
| Humacyte vessel made from regenerative medicine technology. -- Humacyte photos |
Humacyte, a regenerative medicine company based in Morrisville, has received a $9.9 million investment from the California Institute for Regenerative Medicine (CIRM) to support a clinical trial of its potential treatment for kidney disease.
The phase three trial will test the effectiveness of Humacyl, a bioengineered vascular tissue that can be implanted in the arms of patients with end-stage renal disease who are undergoing hemodialysis, or blood purification, a job normally done by healthy kidneys. Humacyl, a tube that functions like a blood vessel, is intended to provide better access to patients’ veins and arteries for extracting and returning blood.
“This approach has the potential to significantly improve our ability to care for people with kidney disease,” said C. Randal Mills, Ph.D., president and chief executive officer of CIRM. “Being able to reduce infections and clotting problems, and increase the consistency of care hemodialysis patients get, would meaningfully impact the quality of their lives.”
The clinical trial will compare Humacyl to the current standard of care, synthetic grafts made from expanded polytetrafluoroethylene.
 |
| Chair, CEO Carrie Cox |
“We’ve achieved a significant milestone for the company with the commencement of our Phase III clinical trial,” said Humacyte Chair and CEO Carrie Cox. “These funds (from CIRM) will greatly help us make strides in the development of our bioengineered human acellular vessel.”
Humacyl received fast track status from the U.S. Food and Drug Administration in 2014, accelerating the regulatory review process.
The clinical trial, which began in May, is being conducted at about 35 sites in the United States, Europe and Israel. It involves about 350 patients who are undergoing hemodialysis but who aren’t suitable for fistula, a surgical solution that joins veins and arteries. The trial is the largest study of any bioengineered vascular tissue to date, the company said.
Humacyte was spun out of Duke University in 2004 by Laura Niklason, M.D., Ph.D., a world leader in tissue engineering.
NCBiotech loan helps propel technology
The company received a $150,000 Small Business Research Loan from the North Carolina Biotechnology Center in 2006. In October 2015 it raised $150 million in a Series B preferred stock financing -- among the largest ever by a life science company in North Carolina.
The privately held company has developed proprietary cell-culture technology for engineering human, extracellular matrix-based tissues that can be shaped into tubes, sheets or particulate conformations, with properties similar to native tissues. These tissues have many potential uses in regenerative medicine and vascular surgery as off-the-shelf products, particularly in patients with vascular disease and those requiring hemodialysis, the company said.
CIRM was created by the State of California in 2004 to accelerate the development of stem cell treatments for patients with unmet medical needs. The agency partners with both academia and industry in a hands-on, entrepreneurial environment to fast track the development of promising stem cell technologies. It has awarded $3 billion in funding and has about 300 active stem cell programs in its portfolio.
