
BioMedomics Gets OK to Sell Sickle Cell Test in Kenya
 |
| Cartons of 20 Sickle SCAN tests each are prepared for shipment by a BioMedomics quality control specialist. -- BioMedomics photos |
Research Triangle Park-based medical diagnostics company BioMedomics is open for business in Kenya while it waits for U.S. Food and Drug Administration approval to sell its medical diagnostics in America.
Frank Wang, Ph.D., CEO, who is also a co-founder of the firm, said BioMedomics has received approval from the Kenya Ministry of Health to sell Sickle SCAN, the company’s portable and quick-reporting test system for sickle cell disease (SCD) and sickle cell trait (SCT). He said BioMedomics has a distributor in Kenya to handle sales there.
SCD is a major public health concern in Kenya because the majority of children born with the disease die before the age of 5. Children who do survive remain vulnerable to painful complications from severe anemia and sickle cell crisis. They also have an increased risk of dying from cardiovascular events and infections.
Kenyan children are not routinely screened for SCD or SCT because of the high cost and limited availability of the complicated central laboratory techniques typically required. As a result, few Kenyans receive treatment for SCD or genetic counseling for those who are carriers of the trait.
SCD is one of the most common genetic disorders, affecting over 100,000 people In the U.S. alone. Most of those who suffer from the painful and life-threatening anemia are African-Americans. Half of this country’s SCD population undergoes frequent blood transfusions.
BioMedomics, started in 2006 with the help of a $19,000 loan from the North Carolina Biotechnology Center, has subsequently received more than $3 million in follow-on funding, including $150,000 in NC IDEA and One NC Small Business state grants and more than $1.5 million in private capital investment.
NCBiotech added to the company’s support earlier in 2016 with a $250,000 Small Business Research Loan.
Sickle SCAN is right for Kenya
Sickle SCAN will provide a cost-effective and easy to use point-of-care test to diagnose both SCD and SCT without the need for any external equipment, power source or trained lab technicians. The device can accurately detect SCD 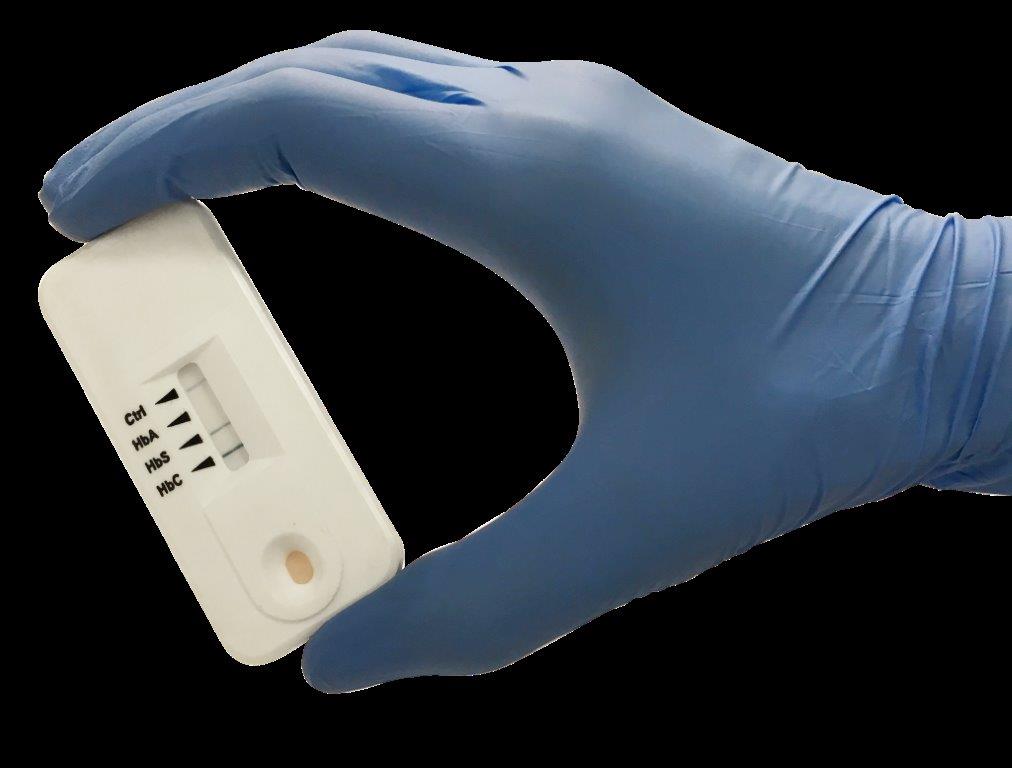 and SCT in five minutes, using only one drop of whole blood from the patient. This simple blood test can be used to dramatically expand Kenya’s efforts to screen newborns for SCD and provide premarital genetic counseling for those who are carriers of the disease.
and SCT in five minutes, using only one drop of whole blood from the patient. This simple blood test can be used to dramatically expand Kenya’s efforts to screen newborns for SCD and provide premarital genetic counseling for those who are carriers of the disease.
“BioMedomics is proud of its innovative solutions for the prevention and management of important blood diseases,” said Wang. “We are committed to bringing advanced point-of-care tests to deliver better health outcomes for our patients. We are very honored to be working with our partners in Kenya to help end the sickle cell crisis there.”
“That early Biotechnology Center support has enabled us to continue successfully developing our technology with the aid of additional federal and state funding,” he said.
Sickle SCAN is one of several point-of-care diagnostic testing systems developed by BioMedomics.
